The preparation method of betamethasone
A technology of betamethasone and organic solvents, applied in the field of preparation of betamethasone, can solve the problems of excessive waste water and waste gas, complex chemical reactions, low utilization rate of raw materials, etc., and achieve the effect of reducing process waste water and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] One embodiment of the present invention provides a preparation method of betamethasone, comprising the following steps S10-S30.
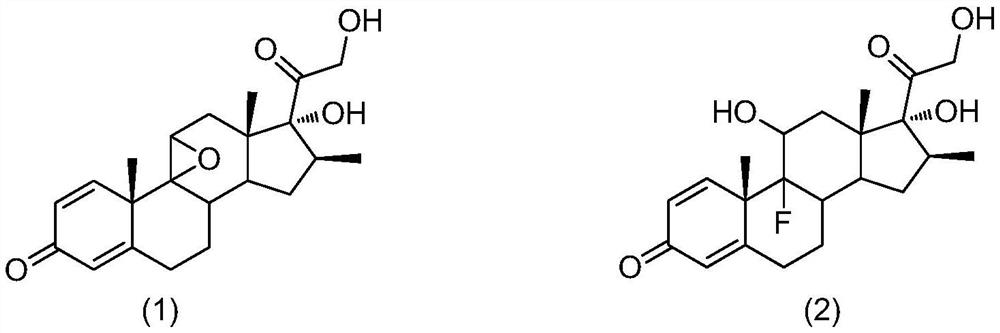
[0038] S10, subjecting betamethasone epoxide and hydrofluoric acid to a ring-opening fluorine reaction to obtain a mixture containing betamethasone;
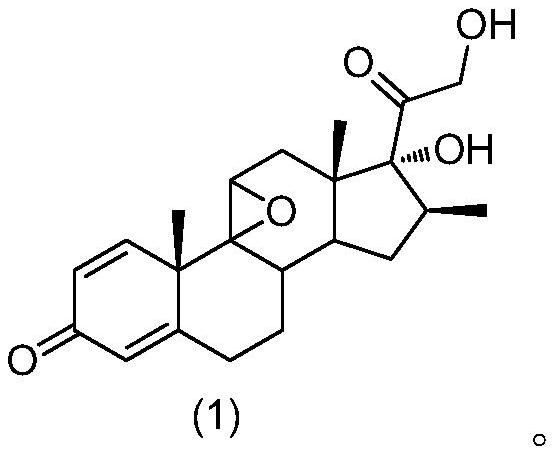
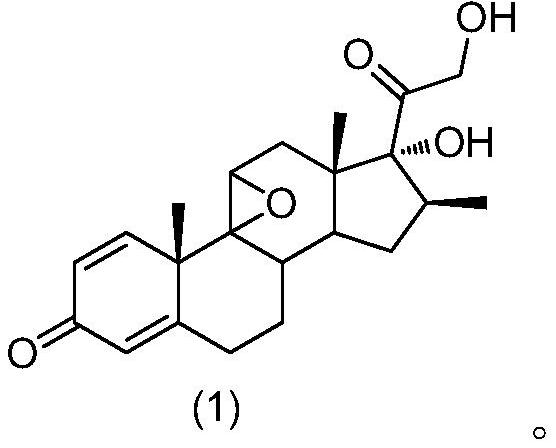
[0039] The structure of betamethasone epoxide is shown in formula (1):
[0040]
[0041] In some of these embodiments, the conditions for the above ring-opening fluorine reaction are: react at -50°C to 0°C for 2h to 5h.
[0042] The ring-opening fluorine reaction is an exothermic reaction, and it is beneficial to reduce side reactions by controlling the reaction at a lower temperature, thereby increasing the yield and purity of betamethasone. Further, the reaction temperature of the above ring-opening fluorine reaction is -35°C to -15°C,
[0043]In some of these embodiments, in the ring-opening fluorine reaction in step S10, hydrofluoric acid is added in the form of hydrofluoric acid aqueo...
Embodiment 1
[0079] 1) Start the reactor to stir, put 5mL ethyl acetate, 1.0g 9β,11β-epoxy-16β-methylpregna-1,4-diene-17α,21-diol-3,20-dione, stir Mix evenly and cool the system down to -35°C, slowly add 0.75mL hydrofluoric acid solution with a mass concentration of 70%, control the adding speed of the hydrofluoric acid solution during stirring, and keep the temperature of the system at -15°C~ -10°C, after the addition is complete, keep the reaction at -15°C to -10°C for 1.5h, and use high performance liquid chromatography (HPLC) to track the raw materials (9β,11β-epoxy-16β-methylpregna-1,4 -diene-17α,21-diol-3,20-dione) <0.1%, the reaction is complete.
[0080] 2) Slowly add the reaction mixture after the reaction in step 1) into the quenching solution, which includes 3 mL of ethyl acetate, 4 mL of methanol and 1.5 mL of water, at a temperature of -5°C, and control the reaction rate by controlling the addition rate of the mixture. The temperature of the system is in the range of -10°C to...
Embodiment 2
[0084] 1) Start the reactor to stir, put 5mL ethyl acetate, 1.0g 9β,11β-epoxy-16β-methylpregna-1,4-diene-17α,21-diol-3,20-dione, stir Mix evenly and cool the system down to -35°C, slowly add 0.75mL hydrofluoric acid solution with a mass concentration of 70%, control the adding speed of the hydrofluoric acid solution during stirring, and keep the temperature of the system at -15°C~ -10°C, after the addition is complete, keep the reaction at -15°C to -10°C for 2 hours, and use high-performance liquid chromatography (HPLC) to track the raw material (9β,11β-epoxy-16β-methylpregna-1,4- Diene-17α,21-diol-3,20-dione) <0.1%, the reaction is complete.
[0085]2) Slowly add the reaction mixture after the reaction in step 1) into the quenching solution, which includes 2.5mL of dichloromethane, 4.5mL of 95% ethanol and 1.5mL of water at a temperature of -5°C. By controlling the mixture The adding speed controls the temperature of the system in the range of -10°C to 5°C. Then slowly add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com