Preparation method of three-dimensional ordered spherical lithium iron phosphate material
A lithium iron phosphate, three-dimensional ordered technology, applied in the field of new energy materials and chemical batteries, can solve the problems of different pore sizes and inconsistent pore types of porous materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
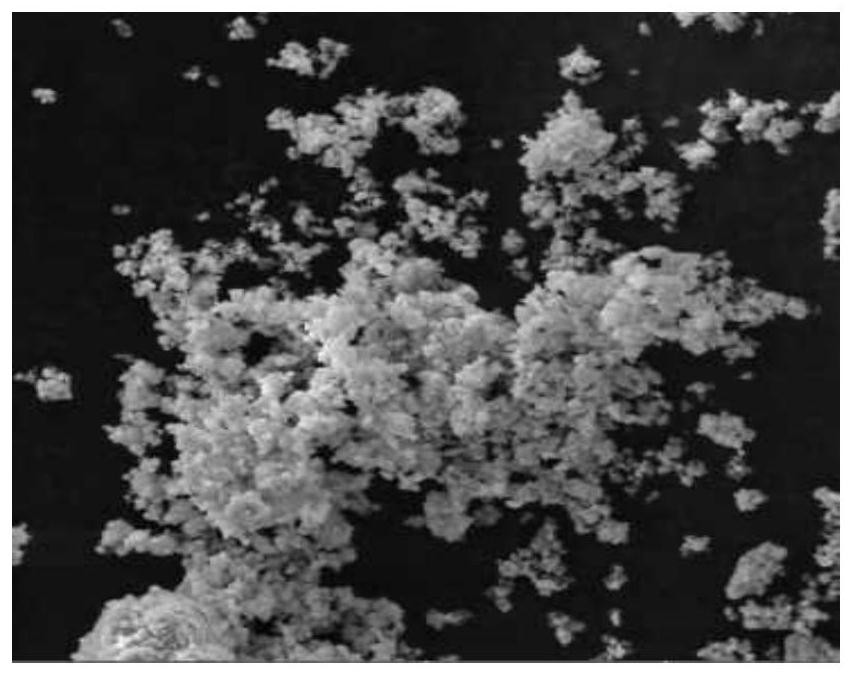
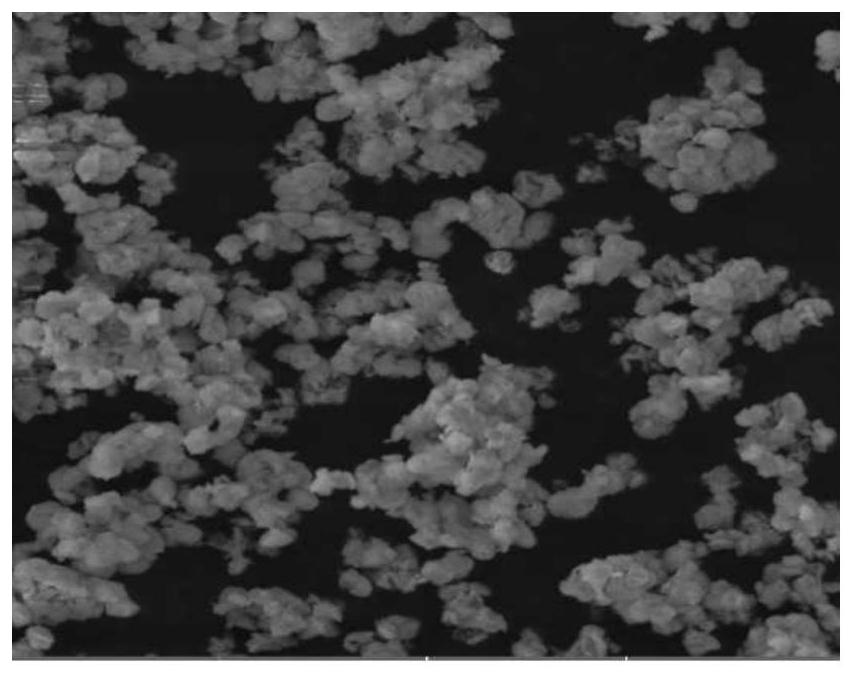
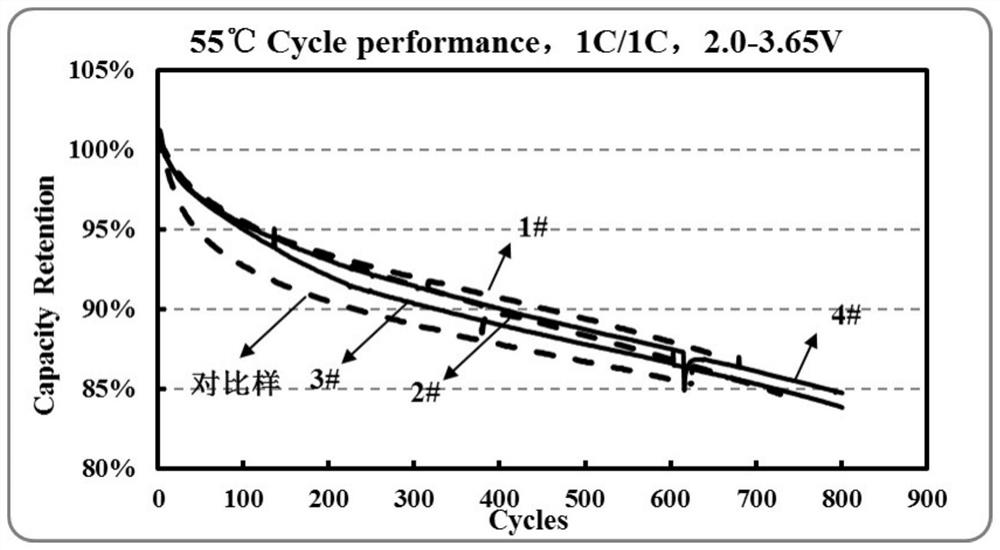
Image
Examples
Embodiment 1
[0027] A method for preparing a three-dimensional ordered spherical lithium iron phosphate material, specifically comprising the following steps:
[0028] (1) Soap-free emulsion polymerization: Add 0.2g potassium bicarbonate and 0.03g sodium styrene sulfonate to 250mL deionized water, stir well to dissolve them completely, transfer them to a 500mL four-necked flask, and then add Add 30mL of styrene monomer into the solution, stir and reflux under nitrogen protection, after the solution is heated to 72°C, slowly add 50mL of a certain amount of initiator potassium persulfate (K 2 S 2 o 8 ) solution, the dropwise addition was completed in 30 minutes, and the closed system reacted for 24 hours after the dripping;
[0029] (2), preparation of lithium iron phosphate precursor sol: under room temperature, iron alkoxide (hexurea iron trinitrate, [Fe(H 2 NCONH 2 ) 6 ](NO 3 ) 3 ) and 10.71 lithium dihydrogen phosphate (LiH 2 PO 4 ) was added to 30ml of absolute ethanol successi...
Embodiment 2
[0034] A method for preparing a three-dimensional ordered spherical lithium iron phosphate material, specifically comprising the following steps:
[0035] (1) Soap-free emulsion polymerization: Add 0.2g potassium bicarbonate and 0.03g sodium styrene sulfonate to 250mL deionized water, stir well to dissolve them completely, transfer them to a 500mL four-necked flask, and then add Add 30mL of styrene monomer into the solution, stir and reflux under nitrogen protection, after the solution is heated to 72°C, slowly add 50mL of a certain amount of initiator potassium persulfate (K 2 S 2 o 8 ) solution, the dropwise addition was completed in 30 minutes, and the closed system reacted for 26 hours after the dripping;
[0036] (2), preparation of lithium iron phosphate precursor sol: under room temperature, iron alkoxide (hexurea iron trinitrate, [Fe(H 2 NCONH 2 ) 6 ](NO 3 ) 3 ) and 10.71 lithium dihydrogen phosphate (LiH 2 PO 4) was added to 30ml of absolute ethanol successiv...
Embodiment 3
[0041] A method for preparing a three-dimensional ordered spherical lithium iron phosphate material, specifically comprising the following steps:
[0042] (1) Soap-free emulsion polymerization: Add 0.5g potassium bicarbonate and 0.03g sodium styrene sulfonate to 250mL deionized water, stir well to dissolve them completely, then transfer them to a 500mL four-necked flask, and then add Add 30mL of styrene monomer into the solution, stir and reflux under nitrogen protection, after the solution is heated to 72°C, slowly add 50mL of a certain amount of initiator potassium persulfate (K 2 S 2 o 8 ) solution, the dropwise addition was completed in 30 minutes, and the closed system reacted for 24 hours after the dripping;
[0043] (2), preparation of lithium iron phosphate precursor sol: under room temperature, iron alkoxide (hexurea iron trinitrate, [Fe(H 2 NCONH 2 ) 6 ](NO 3 ) 3 ) and 10.71 lithium dihydrogen phosphate (LiH 2 PO 4 ) was added to 30ml of absolute ethanol suc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com