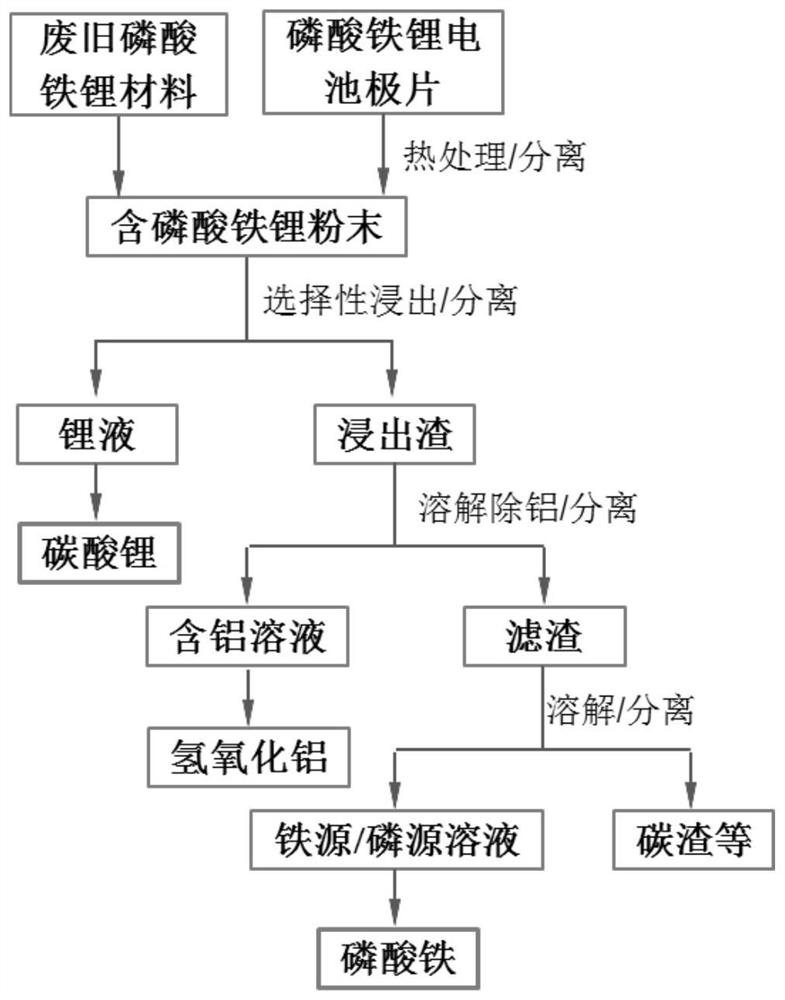
Recovery method of waste lithium iron phosphate positive electrode material
A technology for lithium iron phosphate and positive electrode materials, which is applied in the field of recycling waste lithium iron phosphate positive electrode materials, can solve the problems of containing more aluminum scraps, increasing the cost of impurity removal, and backward recycling technology, so as to achieve high lithium content, reduce purification and Wastewater treatment cost, effect of high industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
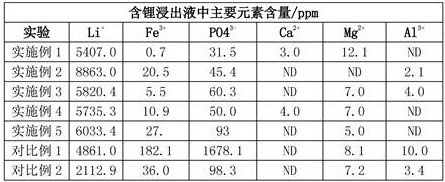
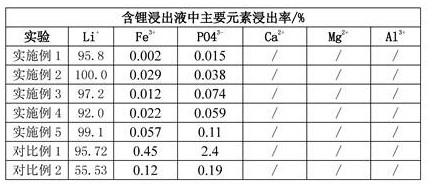
[0064] Separation: ①Treat the crushed lithium iron phosphate-containing pole pieces at 550°C and nitrogen atmosphere for 120 minutes, and then use vibrating sieve and physical separation to separate the current collector from the lithium iron phosphate powder; ②Preparation selectivity Leaching agent A: Add 70g of ammonium sulfate to 320ml of deionized water, then add 25ml of 30wt% hydrogen peroxide solution; and leaching agent B: a mixed solution of 30ml of 3mol / L sulfuric acid solution and 30ml of 30wt% hydrogen peroxide solution③ Put 100g of lithium iron phosphate powder into the prepared leach solution A, and then add the leach agent B dropwise after 5 minutes. The reaction process is greater than 3.0. After stirring for 2 hours, filter to obtain the leach residue and lithium-containing leach solution. The filter cake was washed twice with water to obtain lithium leaching solution. Put the leaching residue into 5% sodium hydroxide aqueous solution, stir and dissolve the al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com