Sustained-release soluble microneedle, preparation method and application
A technology of micro-needle and sustained-release drug, applied in the field of micro-needle, can solve the problems of toxic and side effects, decrease of drug bioavailability, etc., and achieve the effect of slowing down the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a preparation method of sustained-release soluble microneedles, which specifically includes the following steps:
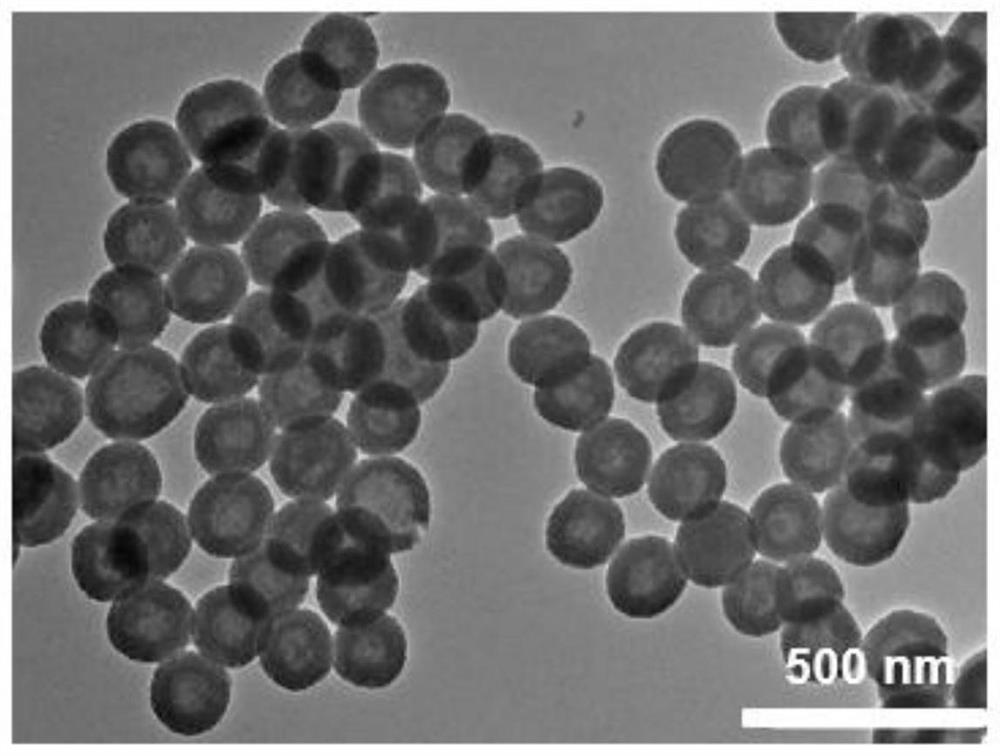
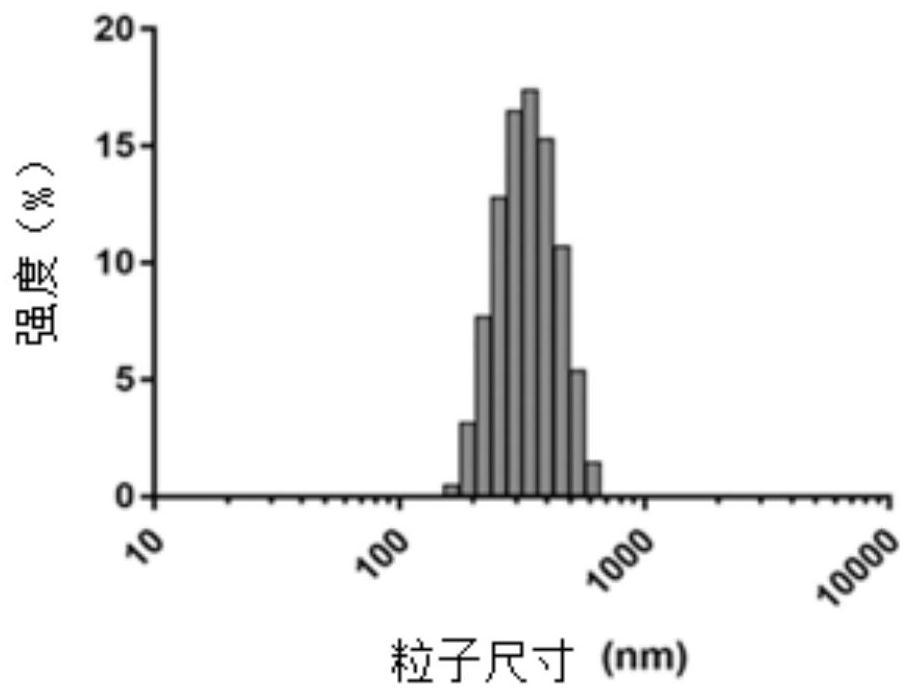
[0034] (1) Preparation of the carrier coated with methotrexate by spray drying method: dissolving chitosan powder and methotrexate in 0.2% hydrochloric acid aqueous solution, spraying into a desiccator for drying after dissolving completely, collecting and preparing chitosan drug carrier of methotrexate.
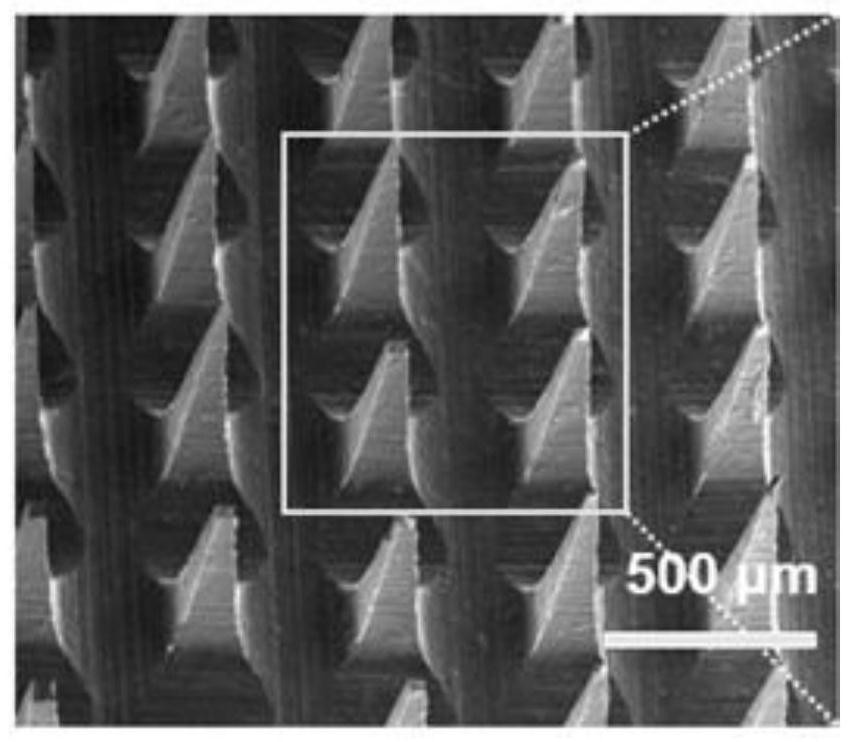
[0035] (2) Microneedle mold preparation: Stir polydimethylsiloxane raw material (PDMS, Sylgard 184) and curing agent evenly at a mass ratio of 10:1, vacuumize to remove air bubbles, and mix the liquid polydimethylsiloxane containing curing agent The base siloxane was cast into a plastic Petri dish with a polymethacrylic microneedle positive film (the length of the positive film needle tip is 850 μm), and heated at 80 degrees Celsius for 2 hours. After the PDMS was cured, the microneedle positive film was carefully The membrane is...
Embodiment 2
[0040] This embodiment provides a preparation method of sustained-release soluble microneedles, which specifically includes the following steps:
[0041] (1) Preparation of methotrexate drug carrier by solvent evaporation method: dissolve a certain amount of polylactic acid-glycolic acid copolymer (PLGA) in dichloromethane, add an appropriate amount of methotrexate to disperse evenly, and pour 1% polyvinyl alcohol (PVA) solution, stirred at room temperature at high speed for 3 minutes to form an O / W emulsion, then continued to stir slowly at room temperature for 4 hours to volatilize the organic solvent, and after the organic solvent volatilized, washed and centrifuged to obtain the methotrexate drug carrier.
[0042] (2) Microneedle mold preparation: Stir polydimethylsiloxane raw materials and curing agent evenly at a mass ratio of 10:1, vacuumize to remove air bubbles, and cast liquid polydimethylsiloxane containing curing agent into Put the polymethacrylic acid microneedle po...
Embodiment 3
[0047] This embodiment provides a preparation method of sustained-release soluble microneedles, which specifically includes the following steps:
[0048] (1) Preparation of methotrexate drug carrier by solvent evaporation method: 20 mg of polyε-caprolactone-polyethylene glycol copolymer (PCL-PEG) and 4 mg of methotrexate were dissolved in 10 mL of dimethylformamide, Under the condition of stirring, slowly drop the solution into deionized water, continue to stir for 10 minutes, then carry out dialysis, wash and centrifuge after 24 hours of dialysis to obtain the drug carrier of methotrexate.
[0049] (2) Microneedle mold preparation: Stir polydimethylsiloxane raw materials and curing agent evenly at a mass ratio of 10:1, vacuumize to remove air bubbles, and cast liquid polydimethylsiloxane containing curing agent into Put the polymethacrylic acid microneedle positive film (the positive film needle tip length is 650 microns) in a plastic petri dish, heat at 80 degrees Celsius fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com