Composite material for cathode catalyst layer of metal-air battery and preparation method and application thereof
A technology of metal-air batteries and composite materials, which is applied in the direction of battery electrodes, fuel cell half-cells, primary battery-type half-cells, circuits, etc. It is not suitable for large-scale application and other problems, so as to accelerate the efficiency of cathode oxygen catalytic reaction, facilitate large-scale production, improve energy density and overall battery performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
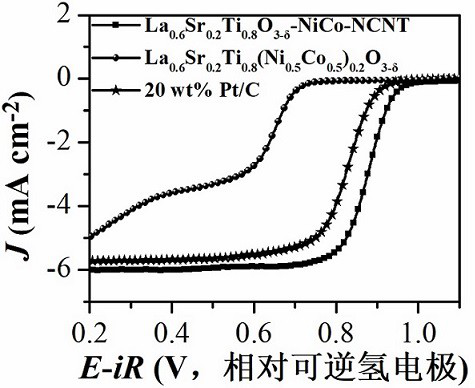
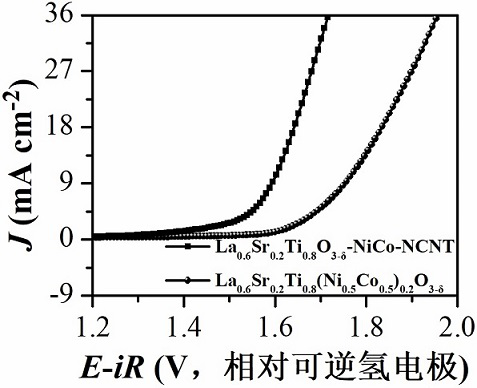
[0075] In an implementation of this embodiment, the specific surface area of the composite material is 200-500 m 2 g -1 . This embodiment also provides a method for preparing the above-mentioned composite material for the cathode catalyst layer of a metal-air battery, comprising: mixing metal-doped perovskite oxide with a nitrogen source, a boron source, a sulfur source, and a phosphorus source At least one and a carbon source, placed in a protective atmosphere for calcination, so that the metal-doped perovskite oxide surface in situ precipitates metal and obtain perovskite oxide, metal catalyst nitrogen source, boron source, sulfur source At least one of the phosphorus source and the carbon source generate doped carbon nanotubes, so as to prepare a composite material for the cathode catalyst layer of the metal-air battery.
[0076]This embodiment is used in the preparation method of the composite material for the cathode catalytic layer of the metal-air battery. When the ...
Embodiment 1
[0088] 1. Metal-doped perovskite oxide La 0.6 Sr 0.2 Ti 0.8 (Ni 0.5 co 0.5 ) 0.2 o 3-δ preparation of
[0089] This embodiment consists of the first component of lanthanum nitrate, the second component of strontium nitrate, the third component of tetrabutyl titanate, and the fourth component of a mixture of nickel nitrate hexahydrate and cobalt nitrate hexahydrate, the above four components Preparation of NiCo-doped perovskite oxide La by sol-gel method 0.6 Sr 0.2 Ti 0.8 (Ni 0.5 co 0.5 ) 0.2 o 3-δ , the specific preparation process is as follows:
[0090] First, tetrabutyl titanate and citric acid monohydrate were dissolved in deionized water at 85 °C under continuous vigorous stirring. The molar ratio of citric acid to tetrabutyl titanate is 5:1. After a clear solution is formed, the required stoichiometric amounts of lanthanum nitrate hexahydrate, strontium nitrate, nickel nitrate hexahydrate, and cobalt nitrate hexahydrate are added to the above solution.
...
Embodiment 2
[0107] 1. Metal-doped perovskite oxide Pr 0.7 (Ba 0.8 Ca 0.2 ) 0.15 Ti 0.85 (Co 0.75 Fe 0.25 ) 0.15 o 3-δ preparation of
[0108] This embodiment consists of the first component of praseodymium nitrate, the mixture of the second component of barium nitrate and calcium acetate, the third component of tetrabutyl titanate, and the fourth component of the mixture of cobalt nitrate hexahydrate and ferric nitrate nonahydrate, Cobalt-iron-doped perovskite oxide Pr 0.7 (Ba 0.8 Ca 0.2 ) 0.15 Ti 0.85 (Co 0.75 Fe 0.25 ) 0.15 o 3-δ , the specific preparation process is as follows:
[0109] First, dissolve tetrabutyl titanate and citric acid monohydrate in deionized water at 80°C under continuous vigorous stirring. The molar ratio of citric acid to tetrabutyl titanate is 5:1. After a clear solution is formed, then The required stoichiometric amounts of praseodymium nitrate hexahydrate, barium nitrate, calcium acetate, cobalt nitrate hexahydrate, and ferric nitrate nonahyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com