Triazine functionalized silsesquioxane-based hybrid porous polymer, preparation method and application thereof
A technology of silsesquioxane and porous polymers, which is applied in chemical instruments and methods, water treatment of special compounds, organic compound/hydride/coordination complex catalysts, etc. It can solve the problem of limited applicable monomers and harsh reaction conditions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A preparation method of a phenyl-substituted triazine functionalized silsesquioxane-based hybrid porous polymer (3Ph-TSHPP), comprising the steps of:
[0060] (1) Add 0.189g of octavinylsilsesquioxane (OVS) with a cage structure, 0.524g of 2,4,6-tri (4-Bromophenyl)-1,3,5-triazine (3Ph-TA), 30 mL N,N-dimethylformamide, 0.138 g tetrakis(triphenylphosphine)palladium, 0.50 g sodium bicarbonate, room temperature Stir under low temperature for 30 minutes, then heat to 120°C, and react for 72 hours; Drying at 80° C. for 10 hours gave light yellow powder solid I (0.40 g, mass yield 93%).
[0061] (2) Soxhlet extract the obtained solid I with methanol and dichloromethane for 24 hours, and dry the obtained solid under vacuum at 80° C. for 24 hours to obtain 3Ph-TSHPP.
[0062] The specific surface area of the obtained 3Ph-TSHPP of the present embodiment is 555m 2 g -1 , the pore volume is 0.55cm 3 g -1 .
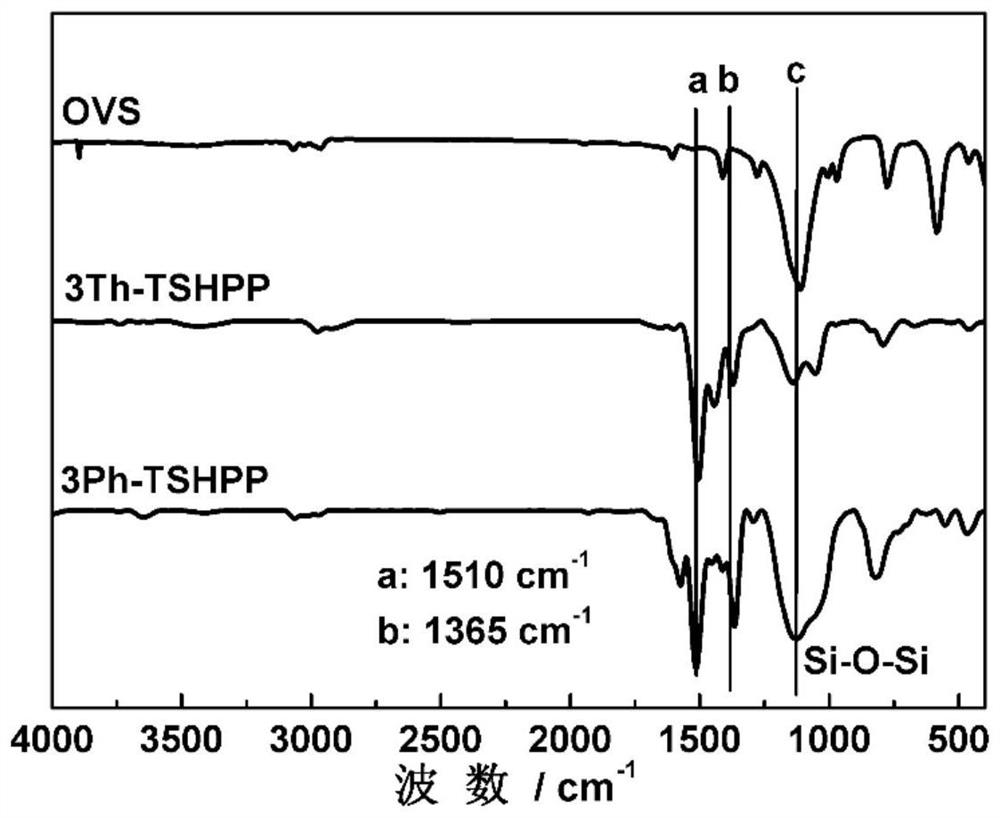
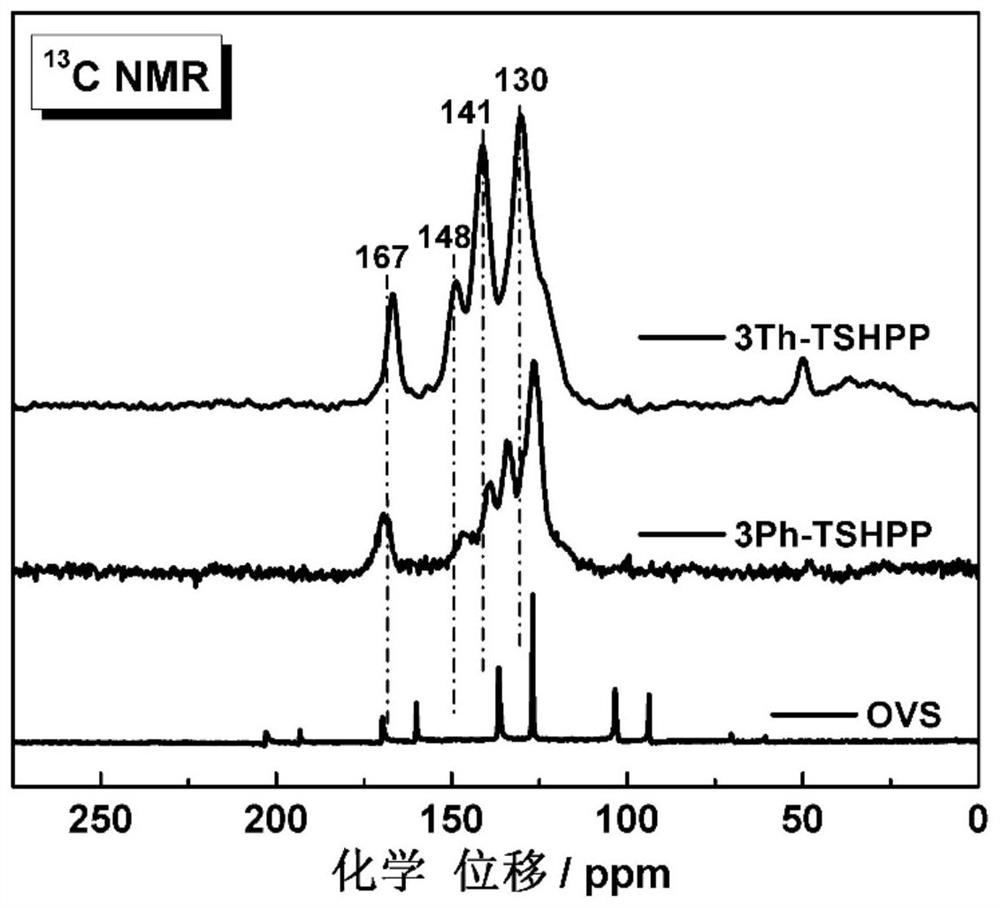
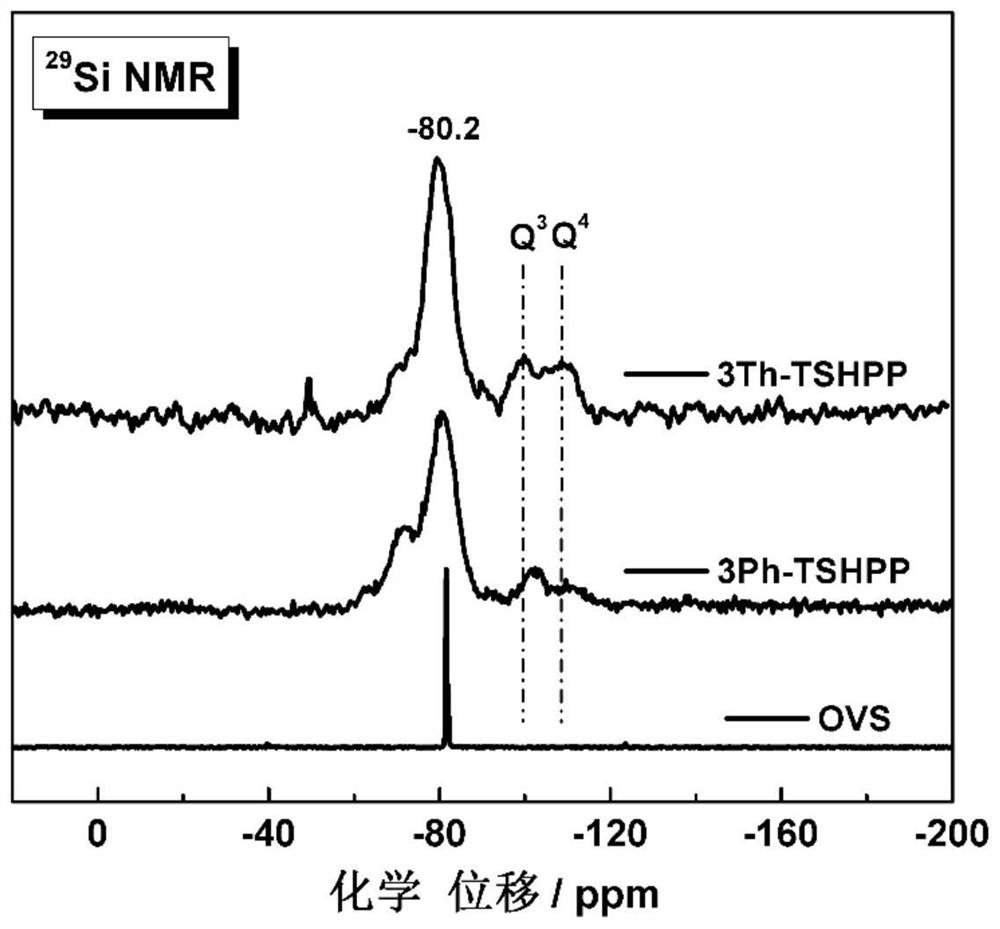
[0063]The infrared spectrograms of octavinylsilsesquioxane (OVS...
Embodiment 2
[0071] A preparation method of a thiophene-substituted triazine functionalized silsesquioxane-based hybrid porous polymer (3Th-TSHPP), comprising steps:
[0072] (1) Add 0.189g of octavinylsilsesquioxane (OVS) with a cage structure, 0.542g of 2,4,6-tri (5-Bromothiophen-2-yl)-1,3,5-triazine (3Th-TA), 30 mL N,N dimethylformamide, 0.138 g tetrakis(triphenylphosphine) palladium, 0.50 g bicarbonate sodium, stirred at room temperature for 30 minutes, then heated to 120°C, and reacted for 72 hours; The second time, drying at 80° C. for 10 hours under vacuum conditions gave brown yellow powder solid II (yield: 0.37 g, mass yield: 82%).
[0073] (2) Soxhlet-extract the obtained solid II with methanol and dichloromethane for 24 hours, and dry the obtained solid under vacuum at 80° C. for 24 hours to obtain 3Th-TSHPP.
[0074] The specific surface area of the 3Th-TSHPP product obtained in this example is 364m 2 g -1 , with a pore volume of 0.40 cm 3 g -1 .
[0075] The infrare...
Embodiment 3
[0083] A preparation method of a phenyl-substituted triazine functionalized silsesquioxane-based hybrid porous polymer (3Ph-TSHPP), comprising the steps of:
[0084] (1) Add 0.189g of octavinylsilsesquioxane (OVS) with a cage structure, 0.437g of 2,4,6-tri (4-bromophenyl)-1,3,5-triazine (3Ph-TA), 30mL N,N-dimethylformamide, 0.055g tetrakis(triphenylphosphine) palladium, 0.50g sodium bicarbonate, Stir at room temperature for 30 minutes, then heat to 120°C, and react for 72 hours; after the reaction, the reaction system is naturally cooled to room temperature, filtered with suction, and the obtained solid is washed three times with methanol, tetrahydrofuran, chloroform, and acetone respectively. Drying at 80° C. for 10 hours under vacuum condition gave light yellow powder solid III.
[0085] (2) Soxhlet extract the obtained solid III with methanol and dichloromethane for 24 hours, and dry the obtained solid in vacuum at 80°C for 24 hours to obtain 3Ph-TSHPP.
[0086] The speci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com