Serum/plasma metabolism molecular marker related to auxiliary diagnosis of intrahepatic cholestasis in gestation period and application of serum/plasma metabolism molecular marker
A molecular marker, cholestasis technology, applied in the fields of metabolomics and reproductive medicine, to achieve the effect of high diagnostic value, convenient and easy diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Collection of research samples and data arrangement
[0040] The inventor collected a large number of peripheral blood samples and placental tissue samples from Wuxi Maternal and Child Health Hospital Affiliated to Nanjing Medical University and healthy control pregnant women from March 2017 to October 2018 (the samples used for research were collected during the same period, sampling, Uniform packaging and storage conditions), through the arrangement of the sample data, the inventor selected 100 samples that meet the following standards as experimental samples for metabolomics detection and verification based on the UPLC-Q active MS method:
[0041] (1) Included case group: The diagnostic criteria of ICP refer to the guidelines for the diagnosis and treatment of ICP patients (first edition), and the specific criteria are as follows: 1) Skin itching occurs in the second and third trimesters of pregnancy, or accompanied by varying degrees of jaundice; 2) Laborat...
Embodiment 2I
[0045] Example 2 ICP patients differential metabolic profile analysis
[0046] The above-mentioned 4 eligible ICP cases and 4 healthy controls were tested by metabolomics to obtain relevant results. The specific experimental method is as follows:
[0047] 1.1 Instrument
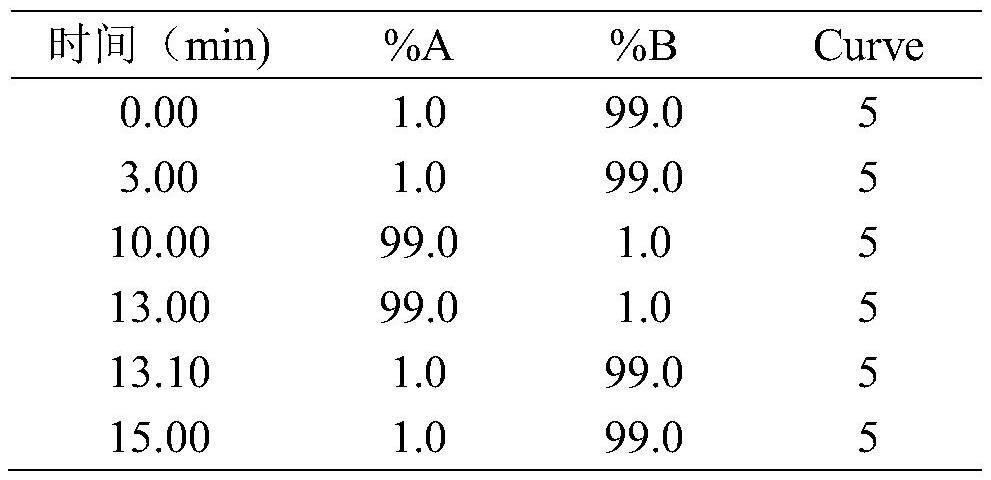
[0048]UPLC Ultimate 3000system (Dionex) high performance liquid chromatography; Q-Exactive triple quadrupole tandem mass spectrometer; TraceFinder 3.1 (Thermo Fisher Scientific); Simca P 13.0 (Umetrics, Sweden); XW-80A vortex mixer (Shanghai Qingpu Hu West Instrument Factory); electronic balance (MettlerAE240); KQ3200B ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.).
[0049] 1.2 Reagents
[0050] 500 metabolite standards (≥98.0%, Sigma–Aldrich, St.Louis, MO, USA); methanol, acetonitrile (≥99.9%, Merck, German); formic acid (≥98.0%, Sigma–Aldrich, St.Louis , MO, USA); n-hexane (chromatographic grade, ≥98.0%, Aladdin Reagent (Shanghai) Co., Ltd.); chloroform (≥99.9%, Alfa Aesar (China) Chemica...
Embodiment 3
[0066] Example 3 Verification of the expression of ICP serum / plasma metabolic molecular markers based on the UPLC-Q exact MS method
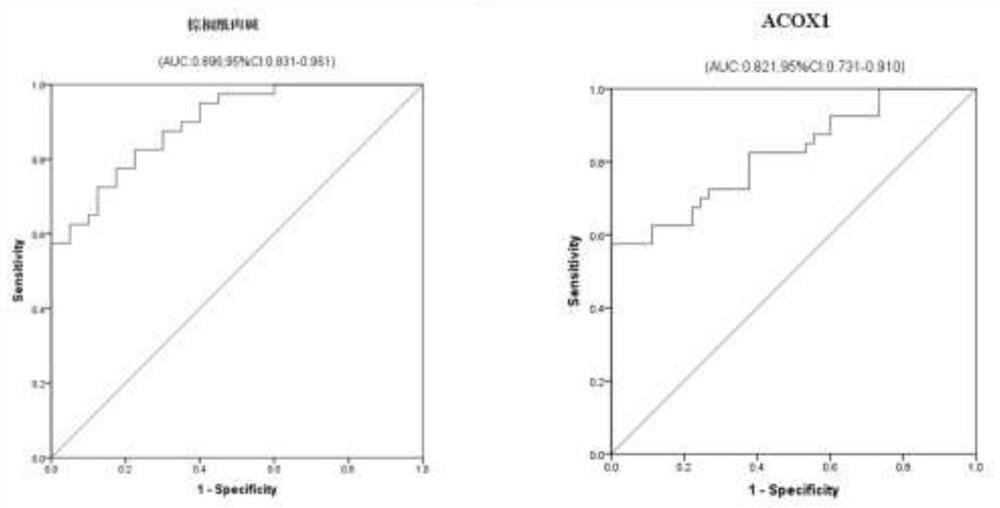
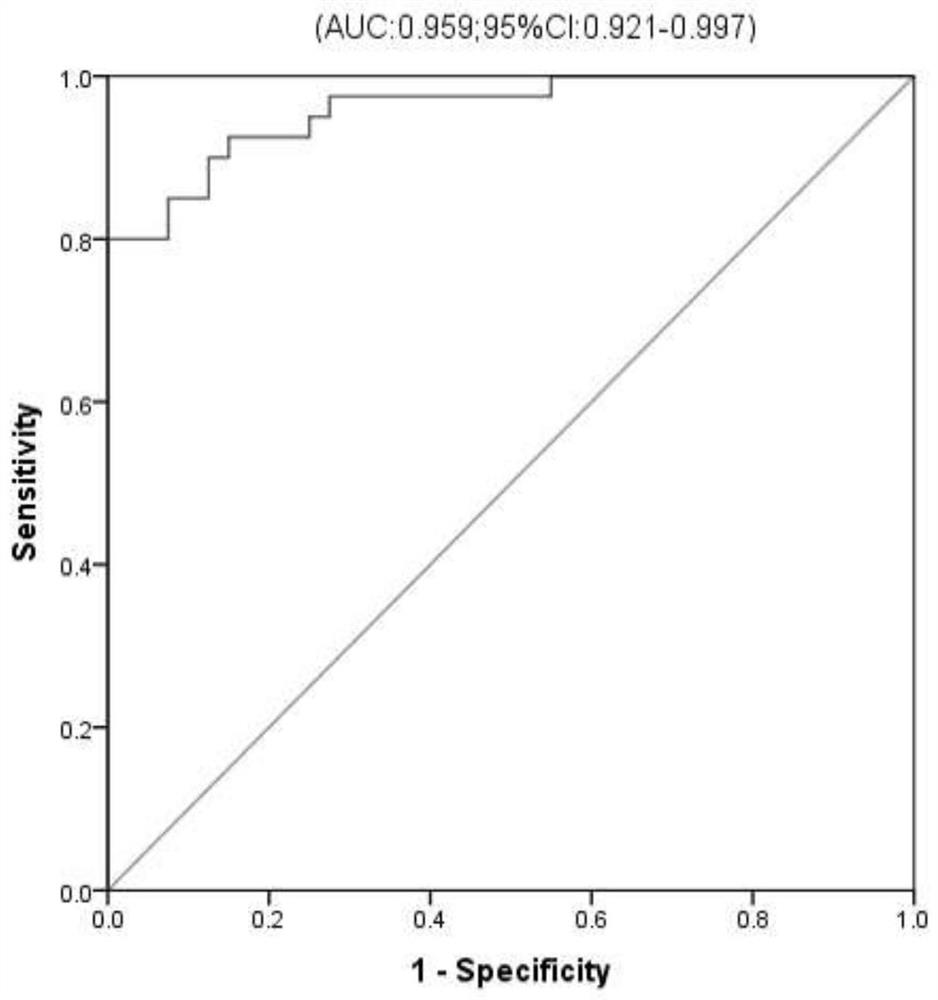
[0067] According to the detection method of UPLC-Q active MS in Example 2, the inventors detected palmitoylcarnitine and ACOX1 in the serum samples of 50 ICP patients and 50 healthy control groups in an independent population, and drew ROC curves accordingly. The demographic characteristics, TBA (μmol / L) level and the average expression levels of metabolic molecular markers palmitoylcarnitine (log mass spectrum peak area) and ACOX1 (pg / ml) in serum / plasma samples were compared by student t test. difference in distribution. Statistical analysis was performed using SPSS16.0 statistical analysis software. The statistical significance level P value was set at 0.05, and all statistical tests were two-sided. The results are shown in Table 1. According to the verification experiments of palmitoylcarnitine and ACOX1 in serum, the inventors detected t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com