Photosensitive compound and preparation method and application thereof
A compound, light-sensitive technology, applied in the field of protein and nucleic acid co-immobilization, can solve the problems of data analysis and interpretation interference, difficult quantitative detection, low signal-to-noise ratio, etc., and achieve the effect of reducing sample loss, small sample volume and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
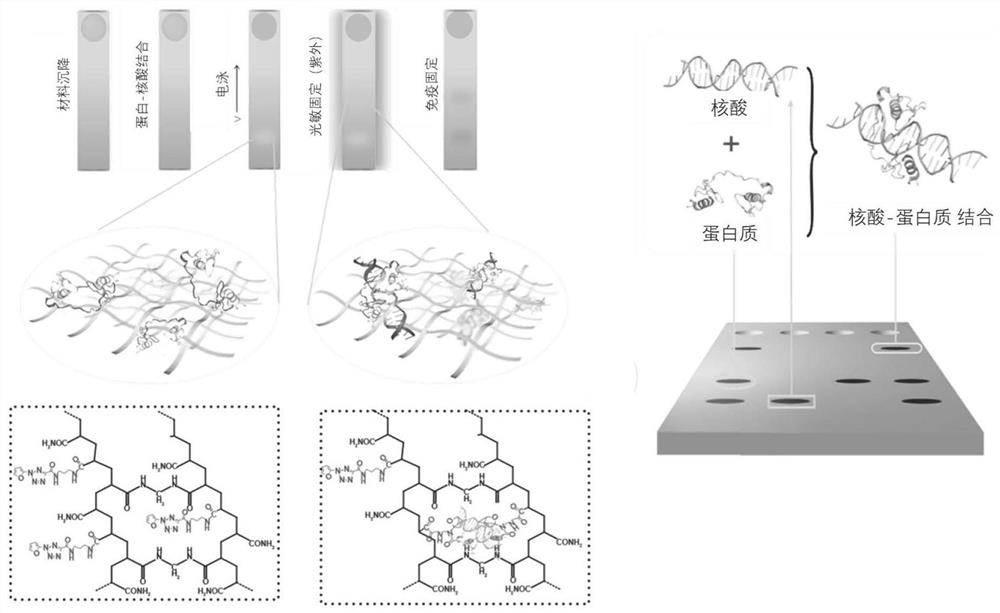
[0080] Example 1: Principle of light-sensitive protein and nucleic acid co-immobilization gel
[0081] The principle of light-sensitive protein nucleic acid co-immobilization gel is as follows: figure 1 As shown, protein-nucleic acid co-immobilization includes the following steps:
[0082] 1.1 Material sedimentation: The purified protein of cells and nucleic acid probes is added dropwise to the gel containing microwells, allowing it to settle into the wells by gravity, and wash away the unsettled cells with PBS;
[0083] 1.2 Protein-nucleic acid binding: cells are cleaved in situ to release protein nucleic acid and incubated to combine protein and nucleic acid probe, nucleic acid and purified protein;
[0084] 1.3 Electrophoresis: Perform non-denaturing gel electrophoresis on a microchip to separate the polymers bound to each other and free monomers;
[0085]1.4 Photosensitive immobilization: the photosensitizer is activated by ultraviolet irradiation, thereby immobilizing p...
Embodiment 2
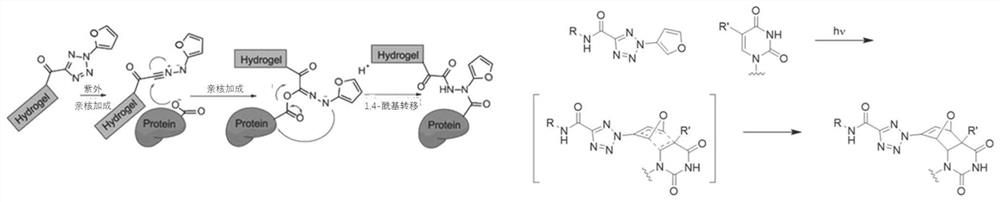
[0087] Example 2: The principle of light-sensitive protein-nucleic acid co-immobilization gel and protein and nucleic acid cross-linking after ultraviolet excitation
[0088] The novel photosensitizer MAP-FTC contained in the light-sensitive protein-nucleic acid co-immobilization gel provided by the present invention is a polyacrylamide compound with both a tetrazole group and a furan group. The principle of light-sensitive protein-nucleic acid co-immobilization gel cross-linking with protein and nucleic acid after ultraviolet excitation is as follows: figure 2 As shown, the chemical formula on the left indicates that the tetrazolyl group of the new photosensitizer MAP-FTC can undergo nucleophilic addition to the protein by the carbon-nitrogen triple bond generated by the tetrazole group of the new photosensitizer MAP-FTC under ultraviolet irradiation at about 346 nm; the chemical formula on the right indicates the furan of the new photosensitizer MAP-FTC The group can underg...
Embodiment 3
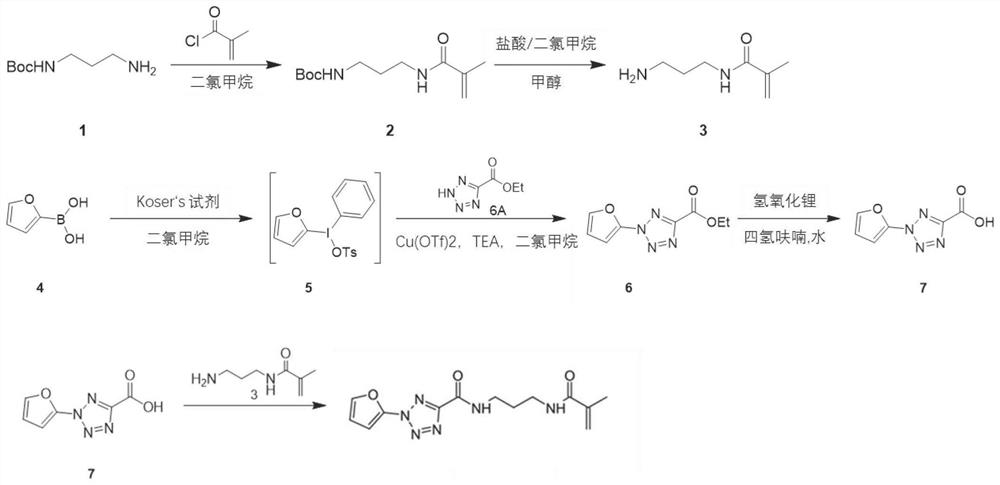
[0089] Embodiment 3: Preparation of light-sensitive compound
[0090] Step 3.1: Preparation of N-[3-(2-methylprop-2-allylamino)propyl tert-butyl carbamate (tert-butyl(3-methacrylamidopropyl) carbamate):
[0091] Such as image 3 As shown in the synthetic route diagram, add methacryloyl chloride to the first compound, ie, tert-butyl N-(3-aminopropyl)carbamate in dichloromethane (DCM), and stir at 15-40°C for 13- 20h, the resulting reaction mixture was treated with NH 4 Cl and brine, washed with anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo; the resulting residue is purified by column chromatography to give tert-butyl(3-methacrylamidopropyl)carbamate as a white solid, i.e. image 3 The second compound shown.
[0092] Step 3.2: Preparation of N-(3-aminopropyl)-2-methyl-prop-2-enamide (N-(3-aminopropyl)-2-methyl-prop-2-enamide):
[0093] Such as image 3 As shown in the synthetic route diagram, to the second compound, i.e., N-[3-(2-methylprop-2-allylamino) propyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com