Photochromic azobenzene polyamide, preparation method thereof and photochromic nylon fiber
An azobenzene polyamide, photochromic technology, applied in the direction of color-changing fluorescent materials, single-component polyamide rayon, single-component copolyamide rayon, etc., can solve the problem of poor spinning processing performance and unsuitable spinning. Silk raw materials, poor processing performance and other problems, to achieve the effects of high yield, simple and reasonable preparation method, and improved high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A preparation method of the above-mentioned photosensitive color-changing azobenzene polyamide, comprising the following steps:
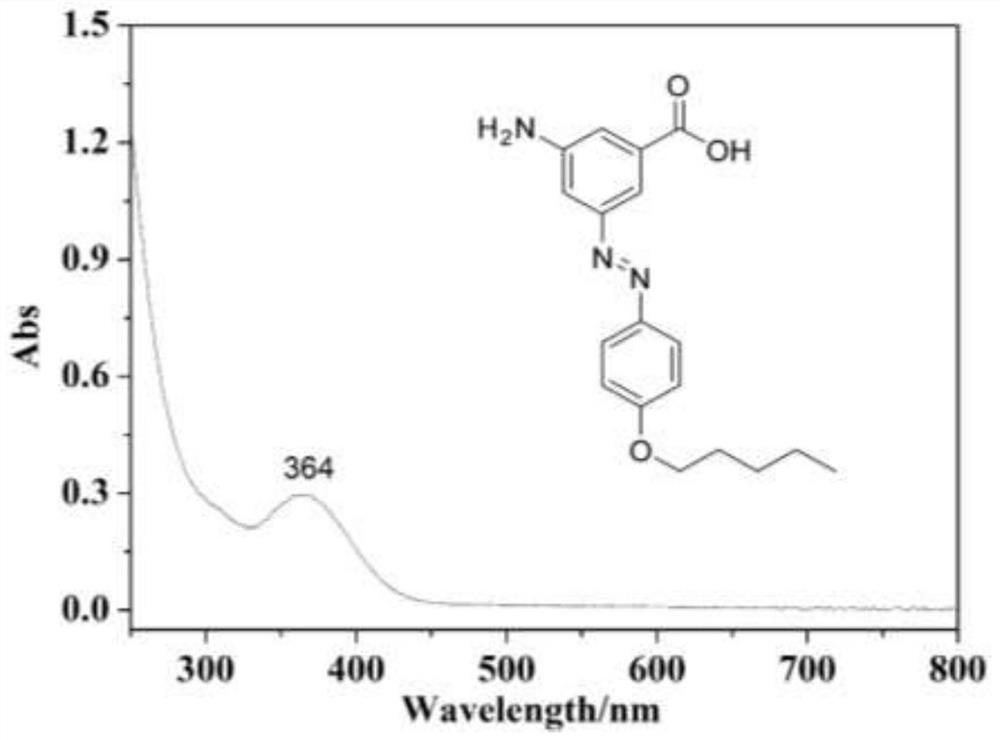
[0043] S1. Preparation of carboxyl-containing azobenzene: 3-amino-5-nitrobenzoic acid suspension is placed in hydrochloric acid solution, the solution is cooled in a water-ice bath at 0-5°C, and the Add aqueous nitrite solution, stir to obtain diazonium salt suspension;
[0044] Wherein, the nitrite is preferably sodium nitrite.
[0045] Then add a solution composed of sodium acetate and phenol, and stir and react at 5°C for 20-40 minutes; then add it to an acidic aqueous solution to obtain a reddish-orange azo compound precipitate;
[0046] Then filter and get the precipitate, use aqueous sodium bicarbonate to wash it, then dry, and recrystallize in boiling n-octane to obtain reddish-orange carboxyl-containing azobenzene;
[0047] S2. Preparation of carboxyl azobenzene containing alkane group: the carboxyl azobenzene obtained in step S1, K...
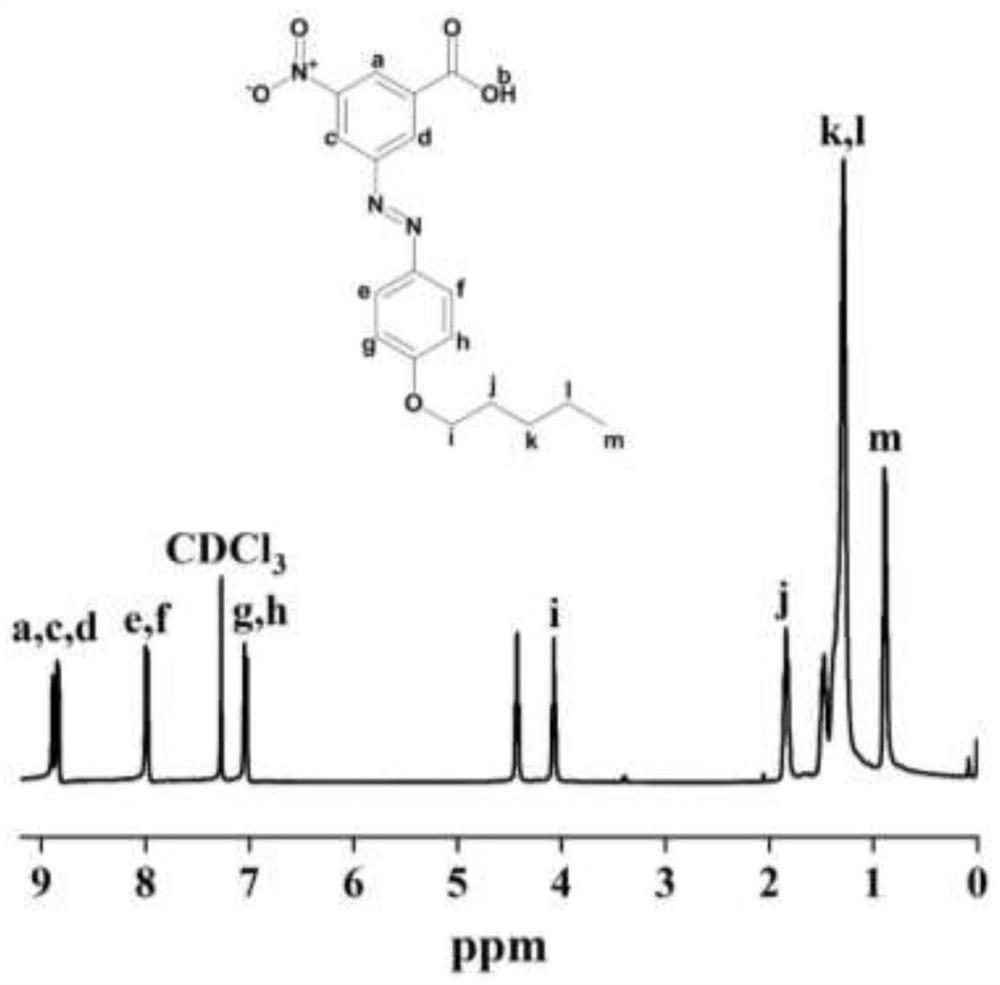
Embodiment 1
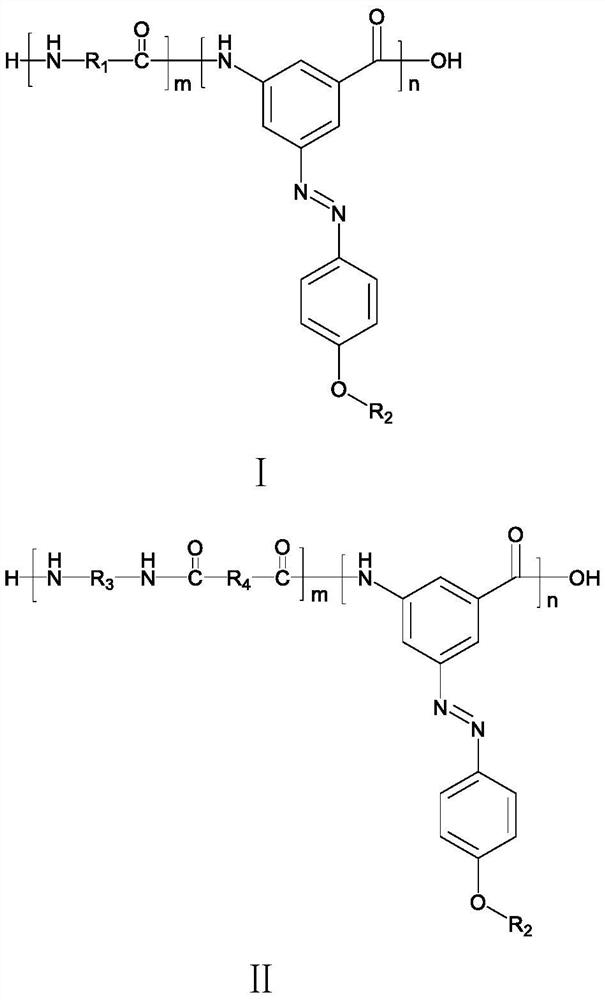
[0062] A photosensitive color-changing azobenzene polyamide, its structural formula is as follows:
[0063]
[0064] Wherein, m is about 330, and n is about 15.
[0065] Prepared by the following steps:
[0066] (1) Synthesis of azobenzene compounds:
[0067] S1. The suspension of 5-amino-3-nitro-1-benzoic acid (9.10 g) was placed in hydrochloric acid solution (300 mL, 0.5 M), and the solution was cooled in a water-ice bath at 0-5°C. Under stirring condition, sodium nitrite (3.80 g) was dissolved in water (solution A) and added slowly. After adding the nitrite solution, continue to stir at low temperature for 20 minutes to finally obtain the diazonium salt suspension;
[0068] Then, sodium acetate (40.8g) and phenol (5.17g) were formulated into a solution (solution B), solution A was added dropwise to solution B, stirred at 5°C, and the system continued to react for 20 minutes; then the final solution was slowly When added to acidic aqueous solution (HCl), a reddish-ora...
Embodiment 2-4 and comparative example 1-2
[0078] The photosensitive color-changing azobenzene polyamides provided by Examples 2-4 and Comparative Examples 1-2, compared with Example 1, are different in that, in step S4, caprolactam and carboxy azobenzene containing alkane groups and amino groups The mass percentage of m 1 :m 2 As shown in Table 1, others are substantially the same as in Embodiment 1, and are not repeated here.
[0079] Preparation conditions and performance test results of table 1 embodiment 1-4 and comparative example 1-2
[0080]
[0081] It can be seen from Table 1 that with the increase of the amount of azobenzene derivatives, the temperature at which the obtained fiber loses 10% of its weight gradually increases. However, because the introduction of azobenzene derivatives reduces the structural regularity of polyamide 6 molecular chains, the tensile strength of the fibers gradually decreases with the increase of its dosage, but the fibers still have the function of UV photochromic change. W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com