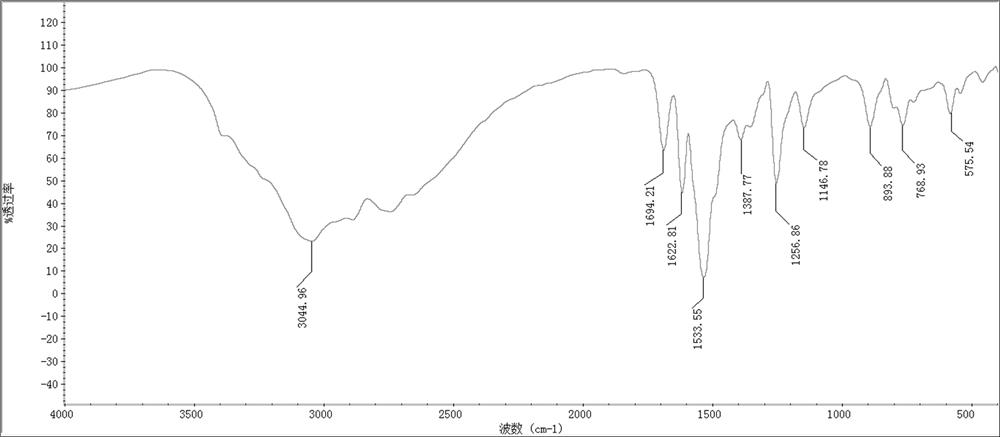
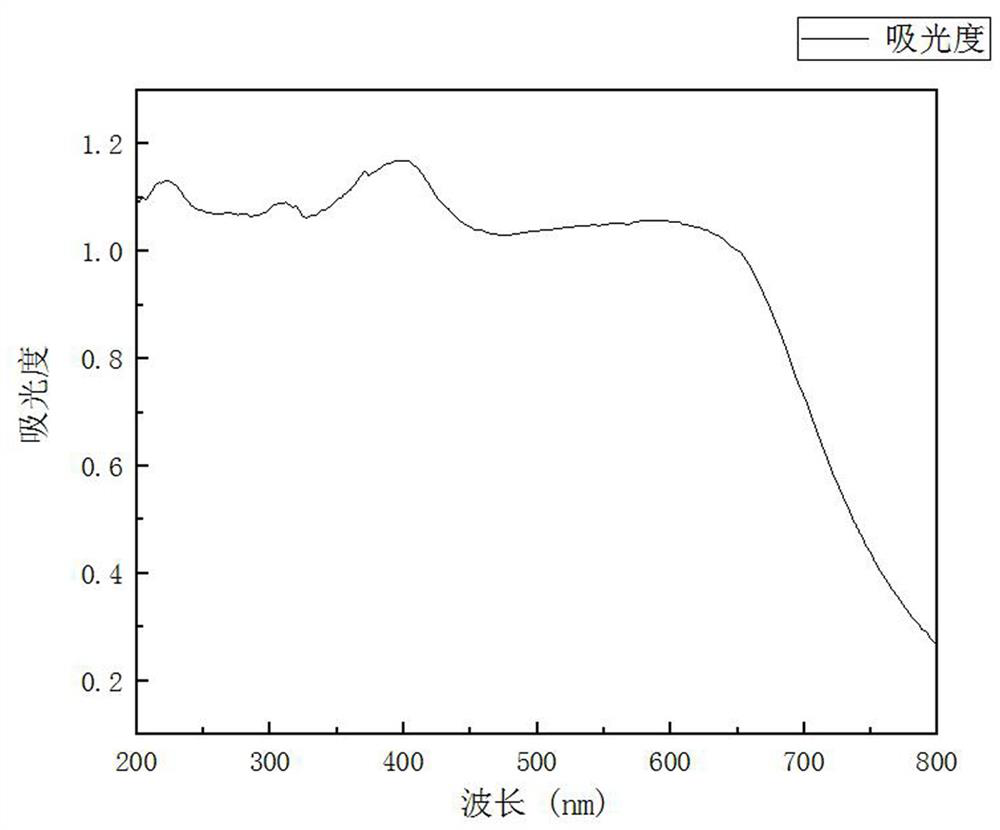
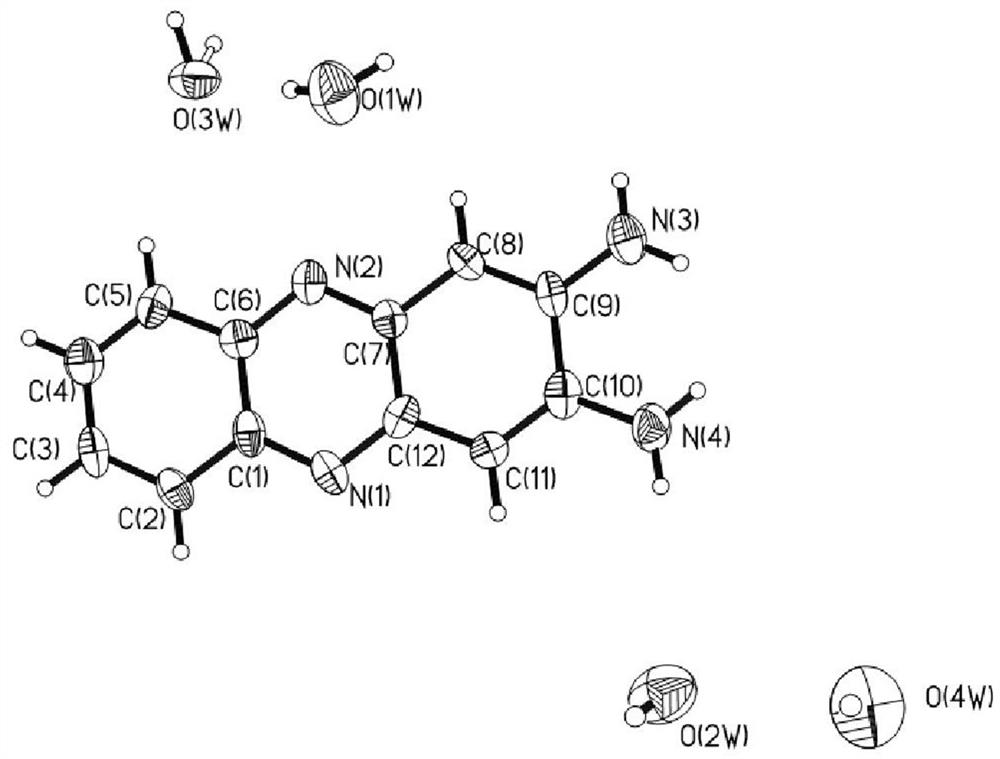
Method for rapidly synthesizing 2, 3-diaminophenazine through ultrasonic radiation catalysis
An ultrasonic and diamino technology, applied in organic chemistry, chemical recycling, resistance to vector-borne diseases, etc., can solve the high price of 2,3-diaminophenazine, increase the production cost of synthetic phenazine, and the process of microbial metabolism. Harsh and other problems, to achieve the effect of expanding the scope of industrial applications, high yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for rapidly synthesizing 2,3-diaminophenazine catalyzed by ultrasonic radiation, comprising the following steps:
[0036] Step 1. Weigh 5.4 g of o-phenylenediamine (50 mmol) and add it to a round-bottomed flask with a volume of 500 mL. Then, dilute 8.35 mL of concentrated HCl (concentration of 12 mol / L) with distilled water to 250 mL. The diluted HCl solution was added to the round-bottomed flask, and the o-phenylenediamine was completely dissolved by magnetic stirring to obtain a raw material solution for subsequent use;
[0037] Step 2, by 60g FeCl 3 •6H 2 O was dissolved in 75 mL of distilled water, then, slowly added dropwise to the above-mentioned raw material solution with a dropping funnel (a red precipitate was formed), after the dropwise addition, the round-bottomed flask was placed in the ultrasonic reactor, and the ultrasonic frequency was set to 60kHZ , under the action of the rated power of 400W, turn on the ultrasonic wave, and carry out the rea...
Embodiment 2
[0044] A method for rapidly synthesizing 2,3-diaminophenazine catalyzed by ultrasonic radiation, comprising the following steps:
[0045] Step 1. Weigh 5.4 g of o-phenylenediamine (50 mmol) and add it to a round-bottomed flask with a volume of 500 mL. Then, dilute 8.3 ml of concentrated HCl (concentration of 12 mol / L) with distilled water to 190 mL. The diluted HCl solution was added to the round-bottomed flask, and the o-phenylenediamine was completely dissolved by magnetic stirring to obtain a raw material solution for subsequent use;
[0046] Step 2, 30g CuCl 2 • 2H 2 O was dissolved in 50 mL of distilled water, then, slowly added dropwise to the above-mentioned raw material solution with a dropping funnel (a red precipitate was formed), after the dropwise addition, the round-bottomed flask was placed in the ultrasonic reactor, and the ultrasonic frequency was set to 65kHZ , under the action of the rated power 400W, turn on the ultrasonic wave, and carry out the reaction ...
Embodiment 3
[0050] A method for rapidly synthesizing 2,3-diaminophenazine catalyzed by ultrasonic radiation, comprising the following steps:
[0051] Step 1. Weigh 5.4 g of o-phenylenediamine (50 mmol) into a round-bottomed flask with a volume of 500 mL, then dilute 8.35 mL of concentrated HCl (concentration of 12 mol / L) with distilled water to 320 mL, and then add The diluted HCl solution was added to the round-bottomed flask, and the o-phenylenediamine was completely dissolved by magnetic stirring to obtain a raw material solution for subsequent use;
[0052] Step 2, put 23.7g NiCl 2 •6H 2 O was dissolved in 60 mL of distilled water, then, slowly added dropwise to the above-mentioned raw material solution with a dropping funnel (a red precipitate was formed), after the dropwise addition, the round-bottomed flask was placed in the ultrasonic reactor, and the ultrasonic frequency was set to 65kHZ , under the action of the rated power 400W, turn on the ultrasonic wave, and carry out the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com