Expression vector of membrane protein AmpG and expression and purification method of membrane protein AmpG
An expression vector, expression and purification technology, applied in the field of protein production, can solve the problems of difficult protein expression and resistance to drug-resistant bacteria, and achieve the effect of improving transcription and translation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The gene cloning of embodiment 1AmpG
[0032] 1. Cloning of AmpG
[0033] a) Design a new AmpG coding sequence (SEQ ID NO.1) based on the codon usage frequency of Escherichia coli in the Kazusa online database (http: / / www.kazusa.or.jp / codon / ) compared to the codons used in the AmpG sequence , and synthesized. Then amplified by PCR method. The PCR reaction system configuration is shown in Table 1.
[0034] Table 1 PCR reaction system preparation table
[0035]
[0036]
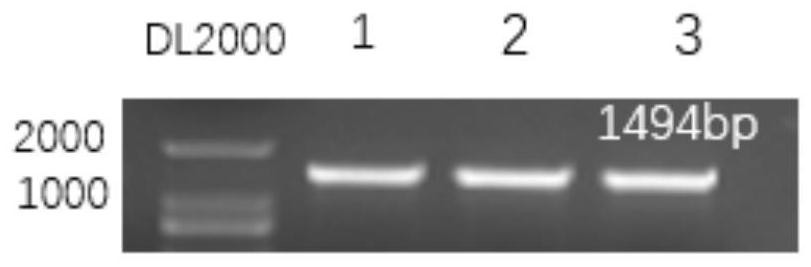
[0037] PCR results such as figure 1 shown. The target fragment length is 1494bp, and the optimal annealing temperature is 52°C.
[0038] b) Recover the target DNA, digest the target fragment and pLy077 vector with NcoI and BamHI, and then perform agarose gel electrophoresis to recover the digested product; add the recovered target fragment and pLy077 vector fragment at a molar ratio of 5:1 In a small centrifuge tube, add T4 ligase and ligate overnight at 16°C.
[0039] c) 20 μL of the above...
Embodiment 2
[0042] The expression of embodiment 2AmpG
[0043] Induced Expression of Fusion Protein AmpG-Superfolded Fluorescent Protein
[0044] The correctly identified pLy077-AmpG plasmid was transferred into Escherichia coli expression host BL21 (DE3) and DH5α, and spread on the colony containing 0.1mM isopropylthiogalactopyranoside (IPTG) to obtain stable transformation; through the detection of colony fluorescence detection, Figure 5 In order to transform the colony fluorescence image on the agar plate, whether the colony contains a recombinant plasmid can be known from the presence or absence of fluorescence, and the colony containing the recombinant plasmid shows fluorescence, and it can be seen that a large number of mutants expressing the target protein have been obtained.
[0045]Inoculate a single colony into LB and TB liquid medium containing 100 μg / ml ampicillin, respectively, and culture overnight at 200 rpm at 37°C to obtain overnight bacteria. Inoculate the overnight b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com