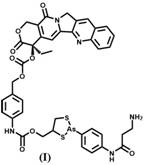
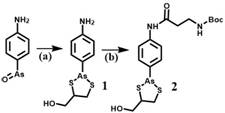
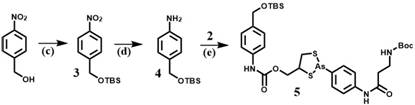
Ortho-dithiol reactive treatment probe for drug release monitoring and preparation method of ortho-dithiol reactive treatment probe
A dithiol and reactive technology, applied in the field of reactive therapeutic probe compounds, can solve problems such as difficult to locate the exact position of the drug, achieve good cell membrane permeability, improve tissue penetration depth, and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing an o-dithiol-responsive therapeutic probe for drug release monitoring, the steps comprising:
[0027] 1) Synthesis of compound 1:
[0028] 4-Aminophenylarsenic oxide (0.29 g, 1.6 mmol) was dissolved in absolute ethanol (20 mL), then 2,3-dimercapto-1-propanol (0.24 g, 1.9 mmol) was added to the solution , the reaction mixture was refluxed for 3 hours, and when the color of the reactant became transparent, the solvent was evaporated under reduced pressure, and column purification (petroleum ether / acetone=5 / 1) gave Compound 1 with a yield of 70%.
[0029] Synthesis of compound 2:
[0030] N-Boc-3-alanine (0.18 g, 0.96 mmol), HATU (0.45 g, 1.2 mmol) and DIPEA (208 μg, 1.2 mmol) were dissolved in anhydrous DMF solution (20 mL), and the reaction system was Stir at room temperature for 0.5-1 hour, then add compound 1 (0.23 g, 0.8 mmol), stir the solution at room temperature for 12 hours, pour into about 800 mL of water, and extract the aqueous phase wi...
Embodiment 2
[0044] Spectral properties of probe CP-VD and its fluorescence response to o-dithiol
[0045] Take the CP-VD synthesized in Example 1 in a test tube, mix it with 4 mL phosphate buffer (pH 4.5) to prepare the probe stock solution (10 μM), then add o-dithiol, and use phosphate buffer to dissolve the final The volume was adjusted to 5 mL. Both absorption and fluorescence spectra were measured in phosphate buffered saline at 37°C. Samples were measured in 1 cm x 1 cm quartz cuvettes (3 mL volume). Absorption measurements were recorded on a UV-Vis spectrometer and fluorescence studies were performed on a fluorescence spectrophotometer with an excitation wavelength of 405 nm, a slit width of 5 nm for both excitation and emission, and a photomultiplier tube detector voltage of 600 V. The free probe CP-VD (10 μM) had an absorbance maximum close to 400 nm and had negligible fluorescence intensity at 37 °C, but upon addition of rBSA (which donates o-dithiol), the maximum absorbance wa...
Embodiment 3
[0047] Specific detection of o-dithiol by probe CP-VD
[0048] Take the CP-VD stock solution (10 μM) in Example 2, after adding the donor of o-dithiol, including reduced human serum albumin (rHSA), rBSA, reduced thioredoxin reductase and thiox The fluorescence of the reduced protein and the probe was significantly enhanced. In contrast, reducing agents, such as dithiothreitol (DTT), low molecular weight proteins, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) and without The proteins with ortho-dithiols had negligible responses to the probe, proving that the fluorescent signal of the probe was caused by the ortho-dithiols.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com