Near-infrared organic supramolecular assembly as well as preparation method and application thereof
A supramolecular assembly, supramolecular polymer technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of fluorescence self-quenching, fluorescence reduction, quenching, etc., and achieve increased fluorescence intensity and biocompatibility. Increased and reduced biotoxicity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of distyryl anthracene derivatives 2Py-DSA and 2Py + -DSA
[0053] Synthesis of 9,10-bis((E)-4-(pyridin-4-yl)styryl)anthracene (2Py-DSA):
[0054]
[0055] Add 0.6mmol of 9,10-bis((E)-4-bromostyrene)anthracene (0.33g), 2.4mmol of pyridin-4-ylboronic acid (0.29g) into 48mL of toluene, and then add 0.03mmol of catalytic Amount of Pd(PPh 3 ) 4 (35mg), in N 2 Stir in the atmosphere for 15min, then add 6mL of 0.8M K 2 CO 3 The aqueous solution and 6 mL of ethanol were mixed evenly, and after continuous reflux reaction in a nitrogen atmosphere for 6.5 h, cooled to room temperature, washed with deionized water three times, and the organic layer was extracted with ethyl acetate and washed with anhydrous MgSO 4 Drying, the precipitate was placed in a vacuum drying oven to dry, and the product with higher purity was obtained by column chromatography, wherein the eluent was dichloromethane (CH 2 Cl 2 ) and ethanol (volume ratio of 9:1), with CH 2 ...
Embodiment 2
[0060] Embodiment 2. Preparation of distyryl anthracene derivatives DSA-(Py + -CH 2 -ph) 2
[0061] Synthesis of (4,4'-(((1E,1'E)anthracene-9,10-diylbis(ethylene-2,1-diyl))bis(4,1-phenylene))bis(1 -Benzylpyridin-1-ium) bromide (DSA-(Py + -CH 2 -ph) 2 ):
[0062]
[0063] Add 9,10-bis((E)-4-(pyridin-4-yl)styryl)anthracene (2Py-DSA) into a mixed solvent of DMF and THF (the volume ratio of DMF and THF is 1:1) , the mixture was heated to reflux until the solution was orange, and (0.2mL, 1.6mmol) benzyl bromide was added dropwise until the solution turned red, and the temperature was raised to 90°C for 6h. After the reaction system produced a large amount of orange precipitate, it was cooled to room temperature. The yellow precipitate was filtered, washed with ethanol and ether, and the solid product was recrystallized in ethanol and acetone to obtain brown-red powder DSA-(Py + -CH 2 -ph) 2 0.14 g, 81% yield.
[0064] 1 H NMR (400MHz, DMSO-d 6 )δ9.24(d, J=6.8Hz, 1H)...
Embodiment 3
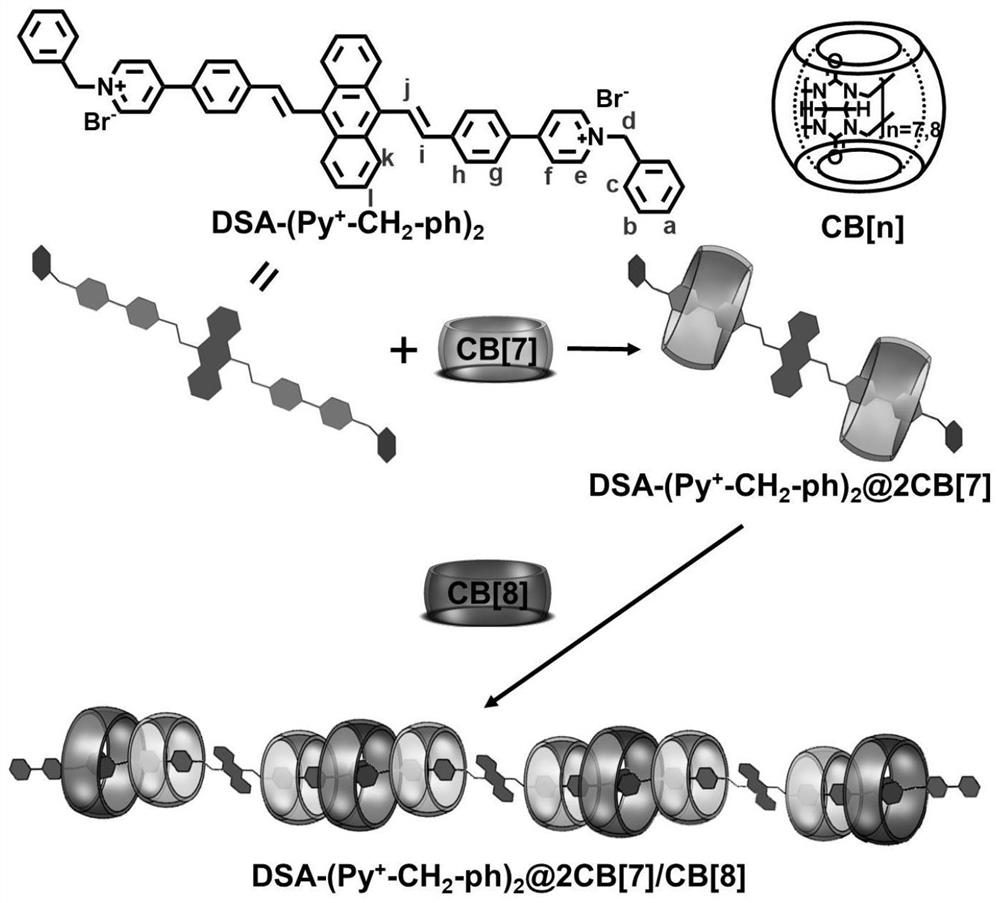
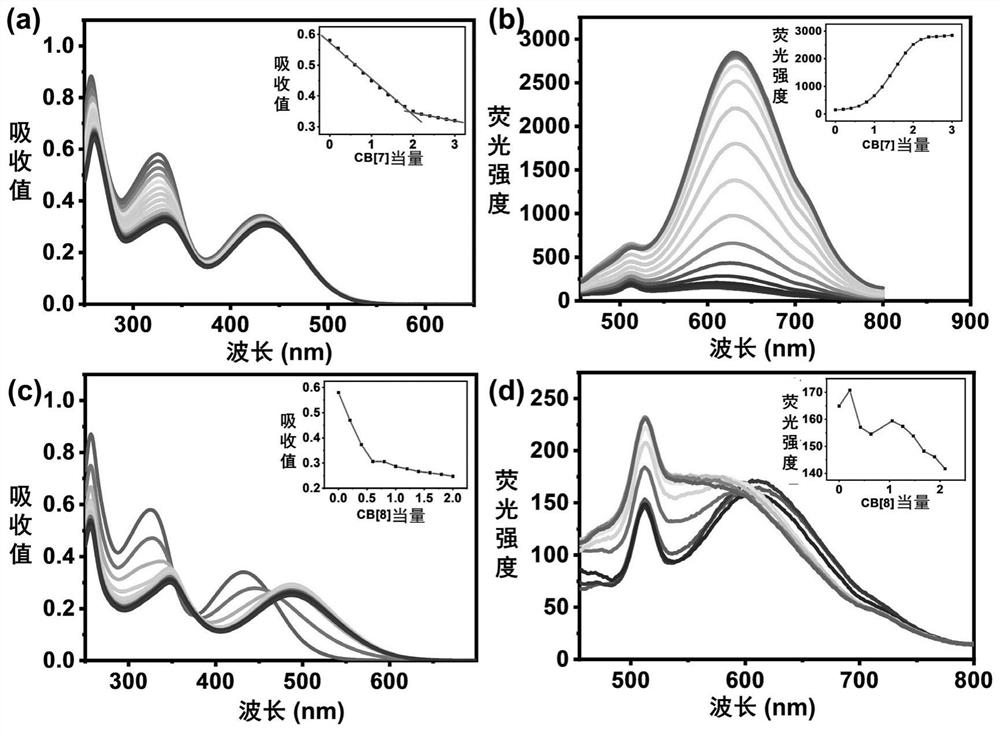
[0066] Preparation of DSA-(Py + -CH 2 -ph) 2 Assemblies with different proportions of cucurbit [7] urea (hereinafter referred to as CB [7]) and cucurbit [8] urea (hereinafter referred to as CB [8]), the specific steps are as follows:
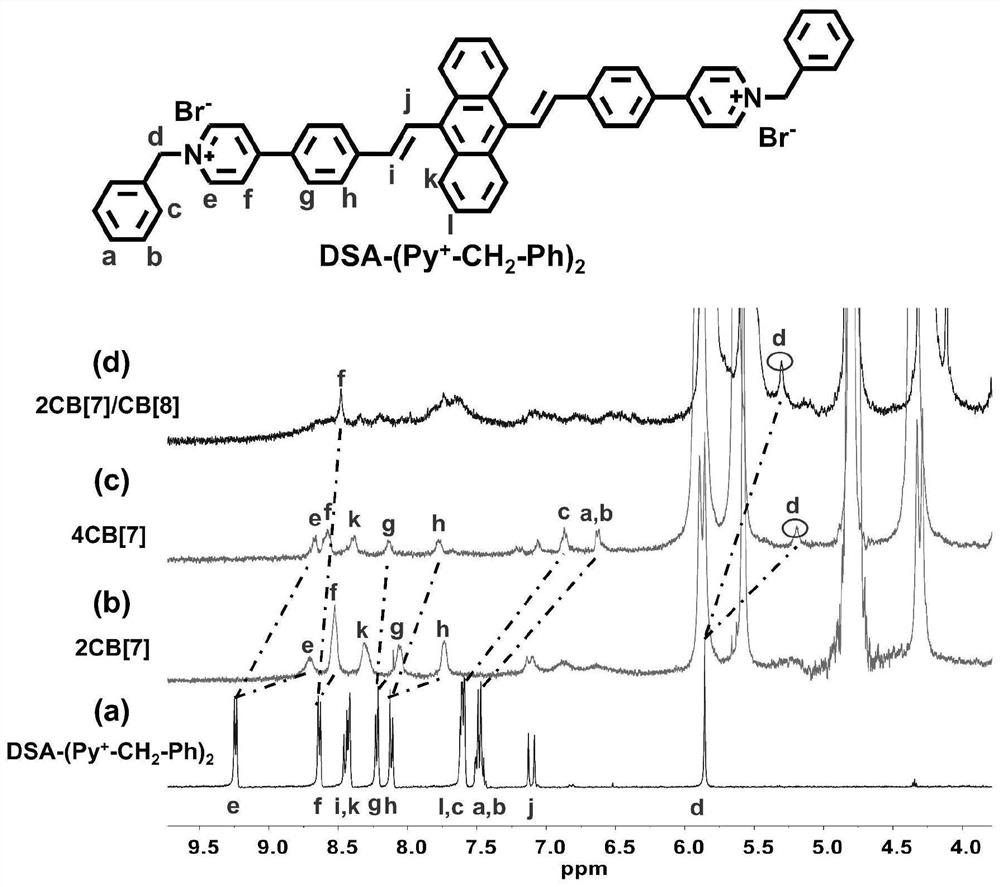
[0067] test(DSA-(Py + -CH 2 -ph) 2 ) in DMSO-d 6 middle 1 H NMR (400MHz) spectrum;
[0068] 1 equivalent of DSA-(Py + -CH 2 -ph) 2 and 2 equivalents of CB[7] were fully assembled in an aqueous solution containing methanol, and a large amount of solvent was removed by rotary evaporation, then dried in a vacuum oven, and then washed with 30% d 6 -DMSO and 70% D 2 O mixed solvent to dissolve it, test its 1 H NMR (400MHz) spectrum (see figure 2 ), CB[7] will DSA-(Py + -CH 2 -ph) 2 The e, f, g, h sites on the package are wrapped to get 2Py + -DSA+2CB[7] assembly;
[0069] 1 equivalent of DSA-(Py + -CH 2 -ph) 2 and 4 equivalents of CB[7] were fully assembled in an aqueous solution containing methanol, a large amount of solvent wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com