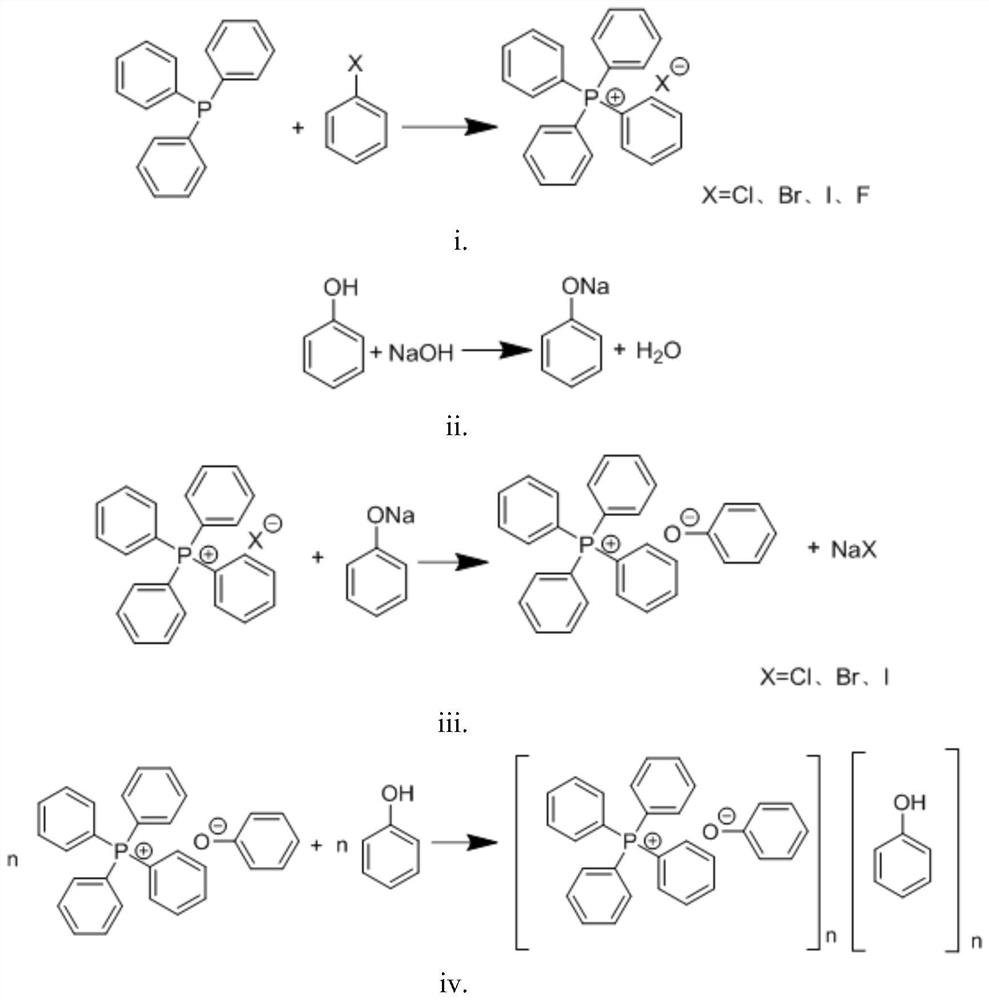
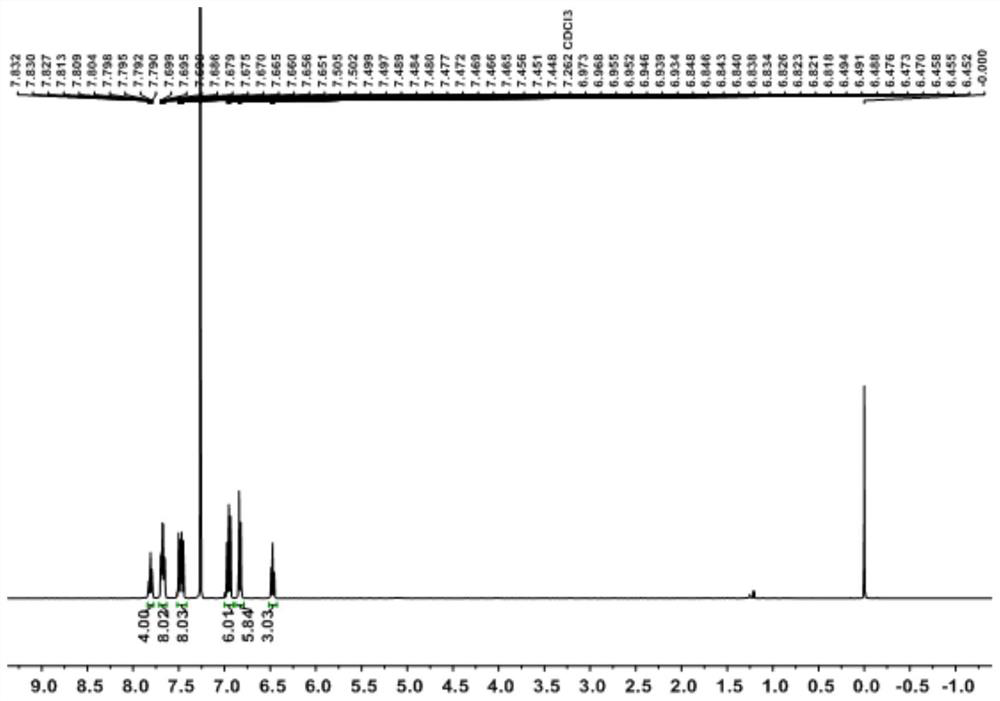
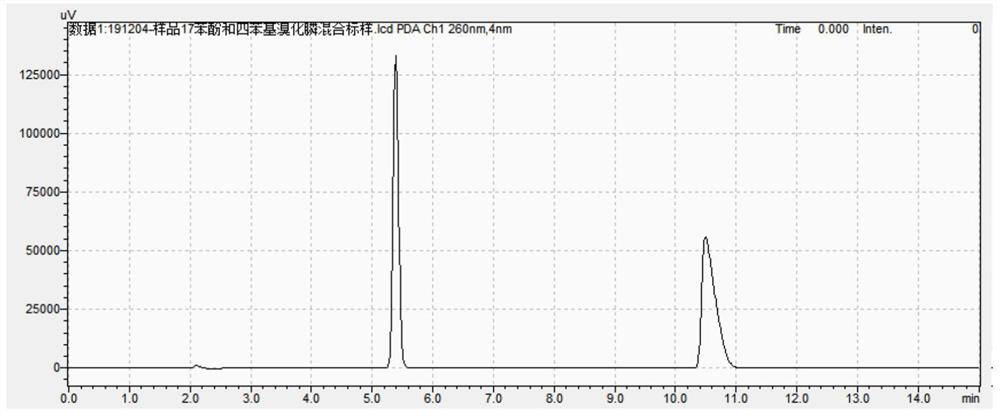
Aqueous phase synthesis method of tetraphenylphosphine phenate
A technology of tetraphenylphosphine phenoxide and tetraphenylphosphine halide, which is applied in the field of aqueous phase synthesis of tetraphenylphosphine phenoxide, can solve the problems of mother liquor recovery and treatment, unstable product components, and inability to continue production. Achieve strong operability and repeatability, easy control of reaction conditions, and avoid massive use and waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of aqueous phase synthetic method of tetraphenylphosphine phenate, concrete steps are as follows:
[0028] In the autoclave, drop into triphenylphosphine (524.5g, 2.0mol), bromobenzene (376.8g, 2.4mol) and catalyst NiBr 2 (4.4g, 0.02mol), the temperature was raised to 160°C, the reaction pressure was 0.3MPa, and a developer was added (the developer was petroleum ether and ethyl acetate with a volume ratio of 2:1), used to soak the TLC plate, and TLC monitoring No remaining triphenylphosphine is the end of the reaction, and tetraphenylphosphine bromide is generated. After the reaction, the temperature was lowered to 100°C, and the excess bromobenzene was recovered by distillation under reduced pressure. During the distillation, appropriate pure water was added to take out the bromobenzene. After bromobenzene is completely distilled, put pure water into the reaction kettle and stir to completely dissolve tetraphenylphosphine bromide, then add sodium hydroxide to a...
Embodiment 2
[0030] In the autoclave, drop into triphenylphosphine (524.5g, 2.0mol), chlorobenzene (337.7g, 3.0mol) and catalyst NiCl 2 (2.6g, 0.02mol), the temperature was raised to 150°C, the reaction pressure was 0.4MPa, and the developer was added (the developer was petroleum ether and ethyl acetate with a volume ratio of 2:1), used to soak the TLC plate, and TLC monitoring No remaining triphenylphosphine is the end of the reaction, and tetraphenylphosphine chloride is generated. After the reaction, the temperature was lowered to 100°C, and the excess chlorobenzene was recovered by distillation under reduced pressure. During the distillation, pure water was added to take out the chlorobenzene, and the recovered chlorobenzene was subjected to liquid separation treatment and applied to the next batch of reactions. After the chlorobenzene is completely distilled, put pure water into the reaction kettle and stir to dissolve the tetraphenylphosphine chloride completely, then add potassium h...
Embodiment 3
[0032] In the autoclave, drop into triphenylphosphine (524.6g, 2.0mol), chlorobenzene (562.8g, 5.0mol) and catalyst PdCl 2 (8.9g, 0.05mol), the temperature was raised to 200°C, the reaction pressure was 1.0MPa, and the developer was added (the developer was petroleum ether and ethyl acetate with a volume ratio of 2:1), used to soak the TLC plate, and TLC monitoring No remaining triphenylphosphine is the end of the reaction, and tetraphenylphosphine fluoride is generated. After the reaction, the temperature was lowered to 110°C, and the excess fluorobenzene was recovered by atmospheric distillation. During the distillation, pure water was added to take out the fluorobenzene, and the recovered fluorobenzene was subjected to liquid separation treatment and applied to the next batch of reactions. After the fluorobenzene is completely distilled, put pure water into the reaction kettle and stir to dissolve the tetraphenylphosphine fluoride completely, then add potassium carbonate to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com