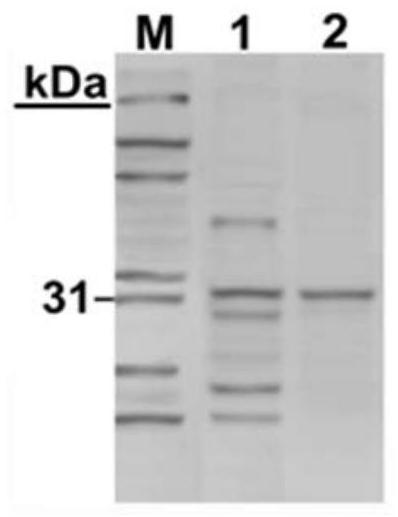
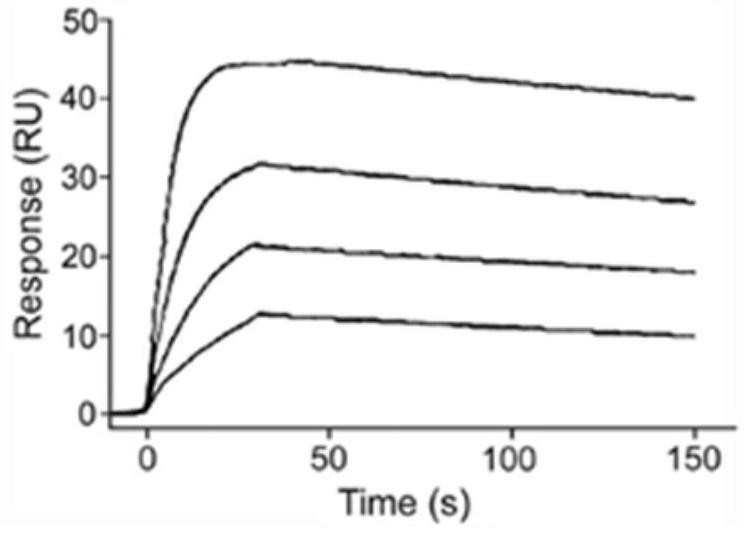
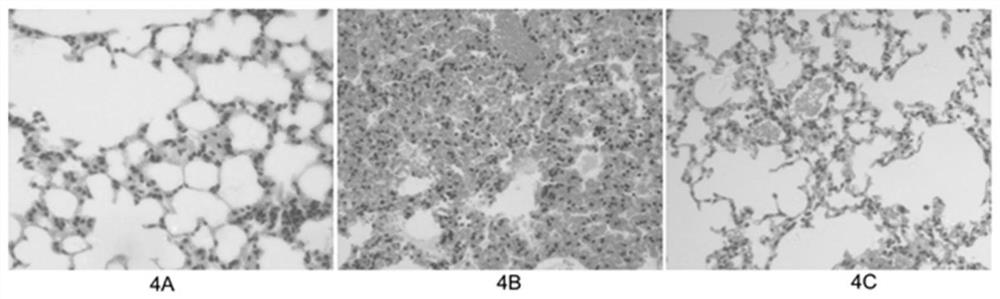
Scaffold proteins derived from epidermal growth factor, lectin and Tat proteins
An epidermal growth factor and scaffold protein technology, applied in the field of protein engineering, can solve the problems of poor stability, low tissue permeability, high production cost, and achieve the effect of high specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Construction of recombinant vector
[0053]First design the 5'-end UTR sequence required for cDNA display: GATCCCGCGAAATTAATACGACTCACTATAGGGGAAGTATTTTTACAACAATT ACCAACAACAACAACAAACAACAACAACATTACATTTTACATTCTACAACTA CAAGCCACCATGGAATTC (including T7 promoter, 5'-UTR sequence of tobacco mosaic virus, kozak sequence and compilation initiation codon sequence, etc.), Named T7Ω. Then design the 3'-end UTR sequence required for cDNA display: TTTCCCCGCCGCCCCCCGCCCTTGTCCGCCAAGCTTGTGATGATGATGGTGGTGAGACCCTCCGCCTGAGCCTCCACCATCATTTGTCCAT CCTTCATC (including 6×His-tag, spacer sequence and linker sequence for connecting puromycin, etc.), named tolA_His. There is a 40bp homologous region between T7Ω and tolA_His, which was sent to a gene synthesis company for synthesis. DNA assembly was carried out according to the operation manual of NEBBuilder HiFi DNA Assembly Master Mix Kit (NEB), and equimolar DNA fragments were added respectively, and then DNA assembly mixture (total...
Embodiment 2
[0054] Embodiment 2: Synthesis of puromycin-biotin cross-linked compound
[0055] Take 20nM puromycin segment (PS): (5'-Thiol-Modifier C6)-TC-(fluorescein-dT)-(12-carbon polyethylene glycol) 4 - CC-Puromycin (BEX), added to 4mM tetrachlorophenol and 50mM phosphate buffer (pH 7.0), mixed at room temperature, desalted with NAP-5 desalting column. Another 10 nM biotin fragment (biotinsegment, BS): CCCGGTGCAGCTGTTTCATC-(Biotin-dT)-CG GAAACAGCTGCACCCCCCGCCGCCCCCCG-(Amino-Modifier C6 dT)-CCT(BEX) and 2 μM 6-(maleimido)hexyl Add acid succinimide ester cross-linking agent to 0.2M phosphate buffer (pH 7.0) and mix well, incubate at 37°C for 30min, and remove excess cross-linking agent by ethanol precipitation at 4°C. The precipitate was washed twice with 70% pre-cooled ethanol, dissolved in 0.2M phosphate buffer (pH 7.0), added PS, and stirred overnight at 4°C. Add 5 mM DTT (dithiothreitol), stir at 37°C for 30 min to stop the reaction, remove excess PS by ethanol precipitation, diss...
Embodiment 3
[0056] Embodiment 3: Preparation of protein-streptavidin (SA) magnetic beads labeled with biotin (biotin)
[0057] SA magnetic beads (Invitrogen) were treated with solution A (diethylpyrophosphate-treated water, 0.1M NaOH, 0.05M NaCl) and solution B (diethylpyrophosphate-treated water, 0.1M NaCl), and added to 2× Binding buffer [20mM Tris-HCl pH 8.0, 2mM ethylenediaminetetraacetic acid (EDTA), 2M NaCl, 0.2% Triton X-100], stored at -20°C for use. Take appropriate amount of coronavirus S protein (Spike) S 1 subunits, N-methyl-D-aspartate receptor (NMDAR), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), epidermal growth factor receptor-2 (HER2), and Dissolve proprotein convertase subtilisin 9 (PCSK9) protein in 0.2M phosphate buffer (pH 7.0), add sufficient amount of Sulfo-NHS-biotin (Pierce), put it on ice for 2 hours, and react to generate Spike-biotin and NMDAR -biotin, VEGF-biotin, TNF-α-biotin, HER2-biotin, and PCSK9-biotin compounds, plus SA m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com