Catalyst for preparing acrylonitrile through propylene ammoxidation as well as preparation method and application of catalyst
A catalyst and acrylonitrile technology, applied in the preparation of hydrocarbon ammoxidation, catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problems of high alkali metal impurity content, gap in catalytic performance, and gelation time Short and other problems, to achieve the effect of increasing the yield of hydrocyanic acid, increasing the amount of lattice oxygen, and improving the processing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A catalyst for producing acrylonitrile by ammoxidation of propylene, the preparation method comprising the steps of:
[0029] 1) After dissolving 3.53 grams of potassium nitrate, 7.81 grams of cesium nitrate, 278.6 grams of ferric nitrate, 522.2 grams of nickel nitrate, 123.5 grams of magnesium nitrate, 156.4 grams of barium nitrate, 5.97 grams of praseodymium nitrate and 109.7 grams of bismuth nitrate in 60ml of water respectively, The resulting aqueous solution was mixed uniformly to obtain Solution I;
[0030] 2) Dissolve 853.7 grams of ammonium molybdate in 100 ml of water, then add 2715.6 grams of silica sol (the particle size of silicon dioxide contained in the silica sol is about 20 nm) to obtain solution II;
[0031] 3) Mix solution I and solution II, adjust the pH of the mixed solution to 4-6, and stir at 80° C. for 30 minutes to obtain slurry I.
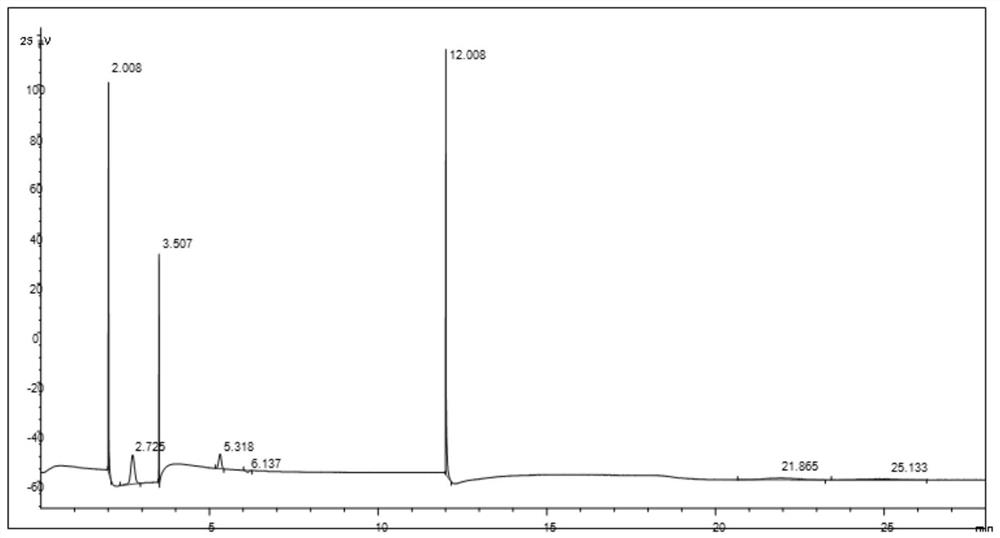
[0032] 4) Slurry I was spray-dried, granulated, and calcined at 620° C. for 3 hours to obtain a catalyst for the ...
Embodiment 2
[0035] 1) After dissolving 3.71 grams of potassium nitrate, 2.82 grams of rubidium nitrate, 8.21 grams of cesium nitrate, 292.53 grams of ferric nitrate, 548.31 grams of nickel nitrate, 129.68 grams of magnesium nitrate, 6.269 grams of praseodymium nitrate and 115.19 grams of bismuth nitrate in 60ml of water respectively, The resulting aqueous solution was mixed uniformly to obtain Solution I;
[0036] 2) Dissolve 896.39 grams of ammonium molybdate in 100 ml of water, then add 2851.38 grams of silica sol (the particle diameter of silicon dioxide contained in the silica sol is about 20 nm) to obtain solution II;
[0037] 3) Mix solution I and solution II, adjust the pH of the mixed solution to 4-6, and stir at 80° C. for 30 minutes to obtain slurry I.
[0038] 4) Slurry I was spray-dried, granulated, and calcined at 620° C. for 3 hours to obtain a catalyst for the ammoxidation of propylene to acrylonitrile.
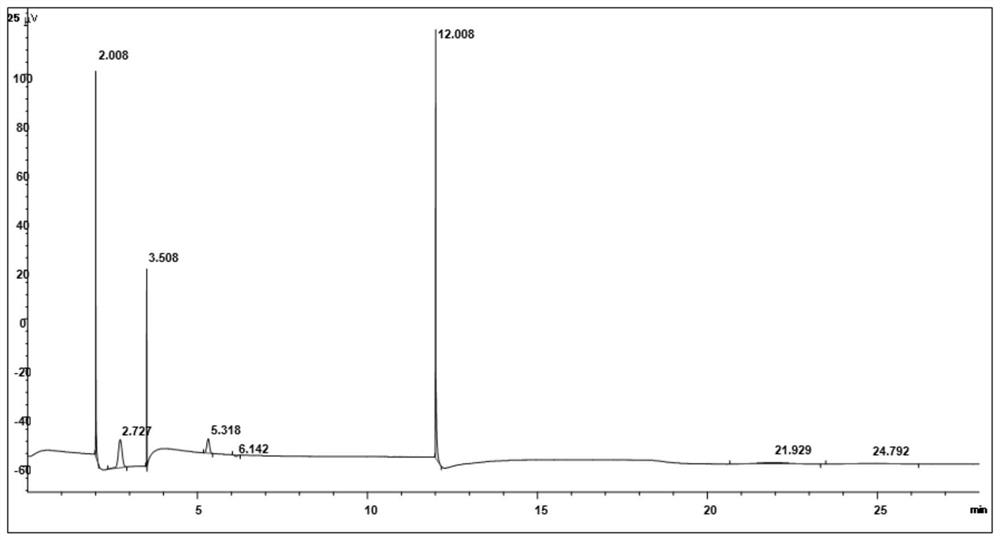
[0039] The resulting product is detected by gas chromatography, and ...
Embodiment 3
[0041] 1) after dissolving 3.60 grams of potassium nitrate, 7.97 grams of cesium nitrate, 284.17 grams of iron nitrate, 532.65 grams of nickel nitrate, 125.97 grams of magnesium nitrate, 73.86 grams of cobalt nitrate, 6.09 grams of praseodymium nitrate and 111.90 grams of bismuth nitrate in 60ml of water respectively, The resulting aqueous solution was mixed uniformly to obtain Solution I;
[0042] 2) Dissolve 870.77 grams of ammonium molybdate in 100 ml of water, then add 2769.91 grams of silica sol (the particle size of silicon dioxide contained in the silica sol is about 20 nm) to obtain solution II;
[0043] 3) Mix solution I and solution II, adjust the pH of the mixed solution to 4-6, and stir at 80° C. for 30 minutes to obtain slurry I.
[0044] 4) Slurry I was spray-dried, granulated, and calcined at 620° C. for 3 hours to obtain a catalyst for the ammoxidation of propylene to acrylonitrile.
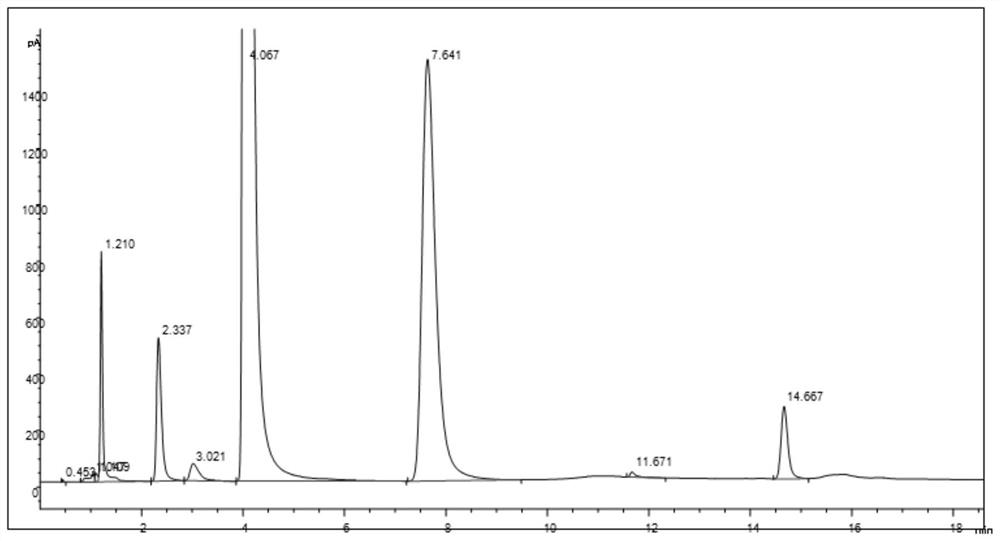
[0045] The resulting product is detected by gas chromatography, and the resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com