Phosphocholine monomer and polymer and application thereof
A technology of choline phosphoric acid and choline phosphoric ester, which is applied in the direction of phosphorus organic compounds, compounds of group 5/15 elements of the periodic table, medical preparations of non-active ingredients, etc., and can solve the problem of phosphatidylcholine groups Difficult synthesis, large-scale production, and functional modification, etc., to achieve excellent conjugation compatibility, high biomimeticity, and promote cell internalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
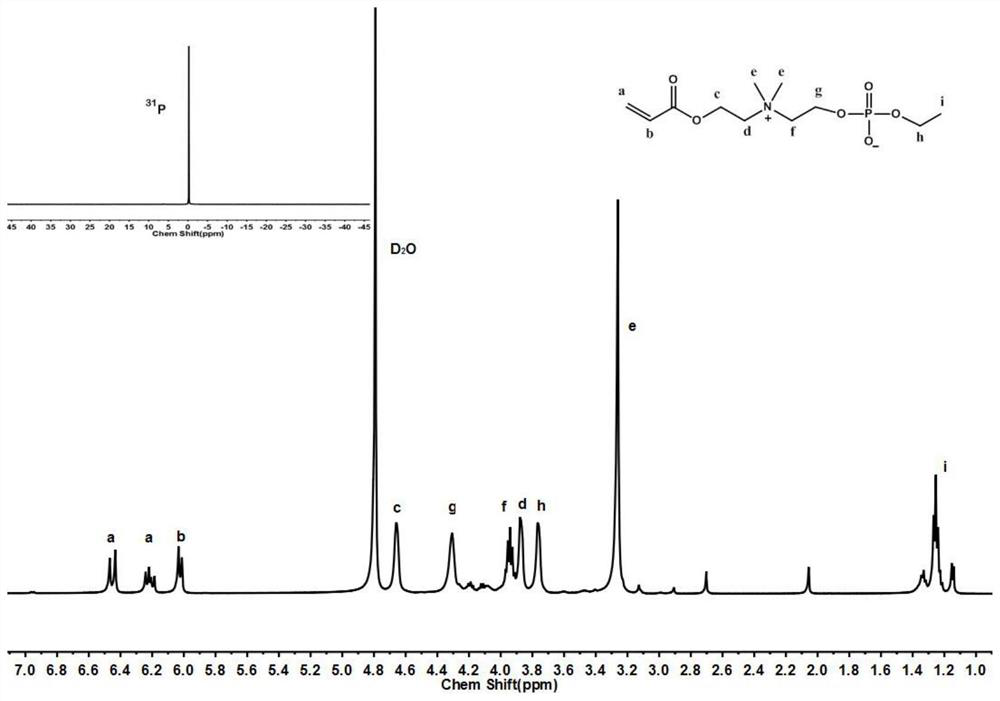
[0063] The preparation of embodiment 1 part acrylic acid choline phosphoric acid ester monomer
[0064] Triethylamine (51g, 0.51mol) and anhydrous methanol (16g, 0.5mol) were added to a branched flask containing 300mL of anhydrous tetrahydrofuran, stirred magnetically under nitrogen protection, cooled in an ice-water bath for 30 minutes, and then Add 2-chloro-2-oxo-dioxaphospholane (71g, 0.5mol) dropwise into the constant pressure dropping funnel. During the dropwise addition, the reaction system produces a large amount of white precipitate, and the dropwise addition is completed within 1 hour. After 2 hours of reaction in a water bath, slowly rise to room temperature, continue to react for 2 hours, filter, wash the filter cake twice with anhydrous tetrahydrofuran, collect the filtrate, remove the solvent under reduced pressure to obtain a crude product, and the crude product is subjected to short-neck distillation to obtain the pure product 2- Oxymethyl-2-oxo-dioxaphospholane...
Embodiment 2
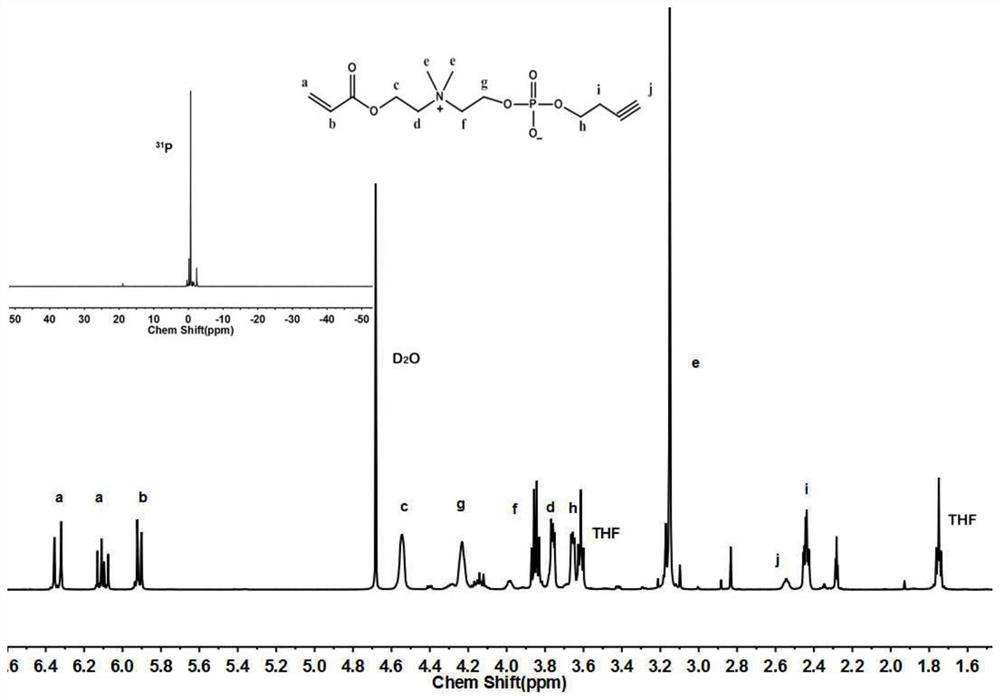
[0078] Preparation of Functionalized Acrylic Phosphate Choline Monomer
[0079] Add triethylamine (51g, 0.51mol) and anhydrous acetylene butanol (35g, 0.5mol) into a branched flask containing 150mL of anhydrous tetrahydrofuran, stir magnetically under nitrogen protection, and cool in an ice-water bath for 30 minutes. 2-Chloro-2-oxo-dioxaphospholane (71g, 0.5mol) was added dropwise from the constant pressure dropping funnel. During the dropwise addition, the reaction system produced a large amount of white precipitate, and the dropwise addition was completed within 1 hour. Keep the reaction in ice-water bath for 2 hours, then slowly rise to room temperature, continue the reaction for 2 hours, filter, wash the filter cake twice with anhydrous tetrahydrofuran, collect the filtrate, remove the solvent under reduced pressure to obtain the crude product, and the crude product is subjected to short-neck distillation to obtain the pure product 44 g of 2-oxobutynyl-2-oxo-dioxaphosphola...
Embodiment 3
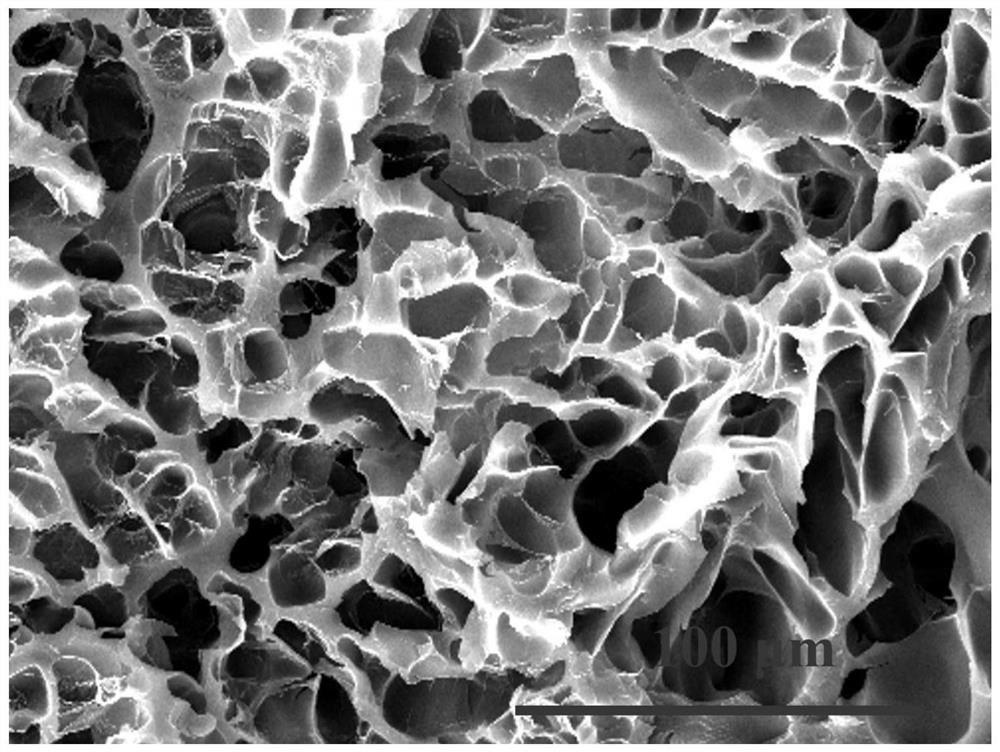
[0086] Preparation and performance characterization of embodiment 3 acrylic acid choline phosphate copolymer and its hydrogel
[0087] The initiator 2-bromo-2-methylpropionic acid 3-azidopropyl ester (2.5mg, 1mmol), 2,2-bipyridyl (0.312g, 2mmol), acryloyloxyethylcholine phosphoric acid ethyl ester (7.03g, 25mmol) was added to a Schlenk bottle containing 30mL of anhydrous methanol, and three times of freezing-thawing-deoxygenation were performed under nitrogen atmosphere, and cuprous bromide (143mg, 1mmol) was added under nitrogen protection. The reaction was carried out at room temperature. After 24 hours, the mixture was exposed to air to terminate the reaction, and then the mixture was dialyzed in deionized water (dialysis bag MWCO 2000). After 24 hours, the sample was freeze-dried to obtain the product PACP25. The product structure is as follows:
[0088]
[0089] 1 H-NMR (500MHz,D 2 O):4.64(t,-CH 2 O-CO-),4.29(t,-CH 2 OP), 3.86(m,-CH 2 N(CH 3 ) 2 -CH 2 -), 3.72...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com