Double-enzyme catalytic system for preparing L-tagatose and application
A catalytic system and tagatose technology, applied in the field of bioengineering, can solve the problems of affecting the catalytic efficiency of enzymes, increasing the difficulty of L-tagatose separation and purification, and increasing the cost of L-tagatose synthesis, so as to reduce the difficulty of purification, Pollution reduction and cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
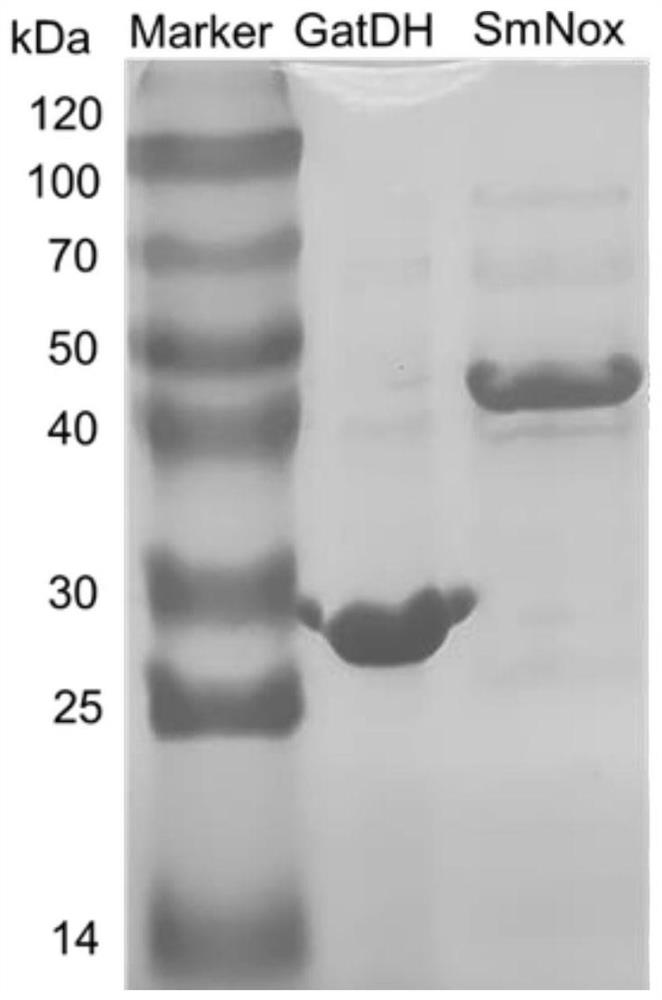
[0026] In this embodiment, the water-based NADH oxidase (SMNOX) derived from the deformation Streptococcus was expressed, and the specific operation steps are as follows.
[0027] Escherichia coli BL21 (DE3) strain with SMNOX recombinant plasmid (E. coli BL21 (DE3) strain is commercialized by Shanghai Freight Biological Engineering Co., Ltd. Reference Literature LI FL, SU WB, TAO QL, et al.EXpression, Biochemical Characterization, And Mutation of a Water Formingnadh: fmn oxidoreductase from Lactobacillus Rhamnosus [J]. Enzyme Andmicrobial Technology, 2020, 134: 109464) Turning with 50 μg · ml -1 The lb medium of kanamycin was overnight, and then expanded in the same 50 μg · ml. -1 Culture in the lb medium of kanamycin. Among them, 50 μg · ml -1 The ratio of lb medium of kanamycin is sodium chloride: trypsin: yeast extract = 1: 1: 0.5, sodium chloride, trypsin and kanamycin are purchased in China Pharmaceutical Group Chemical Reagent Co., Ltd. Yeast extracts were purchased in the l...
Embodiment 2
[0034]In this embodiment, the galactosyl sugar alcohol dehydrogenase (GATDH) of spherical Slim Fungus was expressed, and the specific operation steps are as follows.
[0035] Escherichia coli BL21 (DE3) strain with GATDH recombinant plasmid (E. coli BL21 (DE3) strain is purchased in Shanghai Freight Biological Engineering Co., Ltd., the construction method refers to the literature Zhu Y, LIU CY, CAI S, ET Al. Cloning, Expression and Characterization of a Highly Active Alcohol Dehydrogenase forProduction of Ethyl (S) -4-Chloro-3-hydroxybutyrate [J] Indian Journal ofMicrobiology, 2019, 59 (2):.. 225-233) were plated in 50 μg · mL -1 The 5 ml lb medium of kanamycin was cultured overnight, which was then expanded in the same 50 μg · ml. -1 Culture is carried out in 200 ml lb medium of kanamycin.
[0036] When the bacterial liquid reached 0.9 at 600 nm, it was 0.01 mm, and the final concentration of 0.01 mm was added as an inducer, at 15 ℃ Lower induction 5 h. The high-speed refrigerat...
Embodiment 3
[0040] In this example, the bisase catalytic system is utilized to produce L-Taggosaccharide, and the L-Taggose is measured by cysteine-carbazole to produce production.
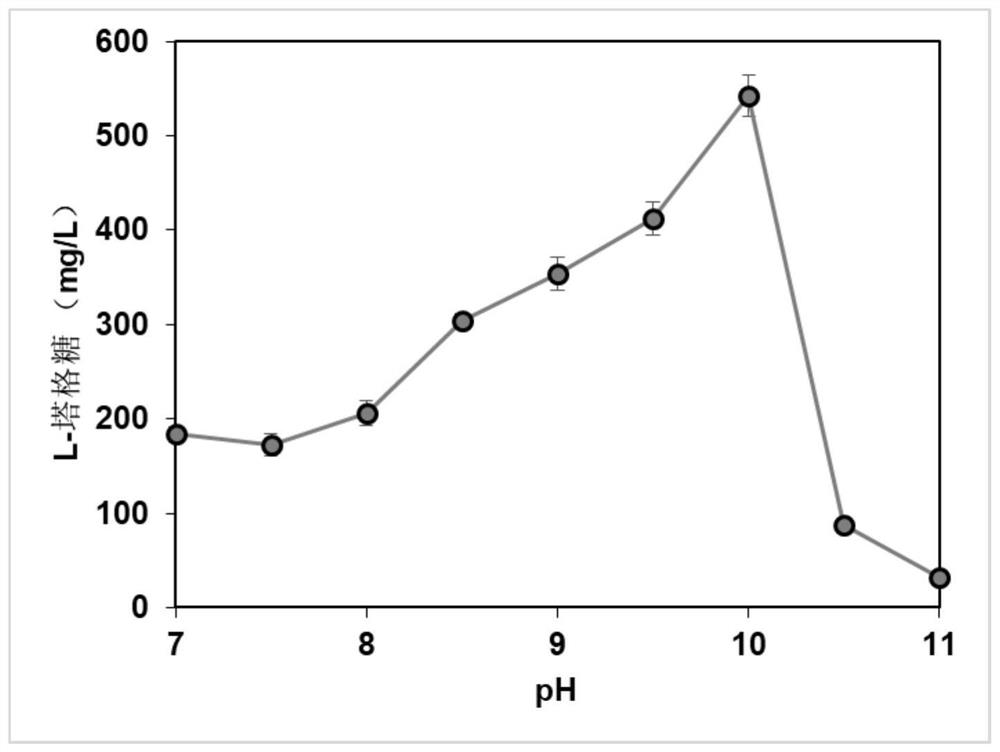
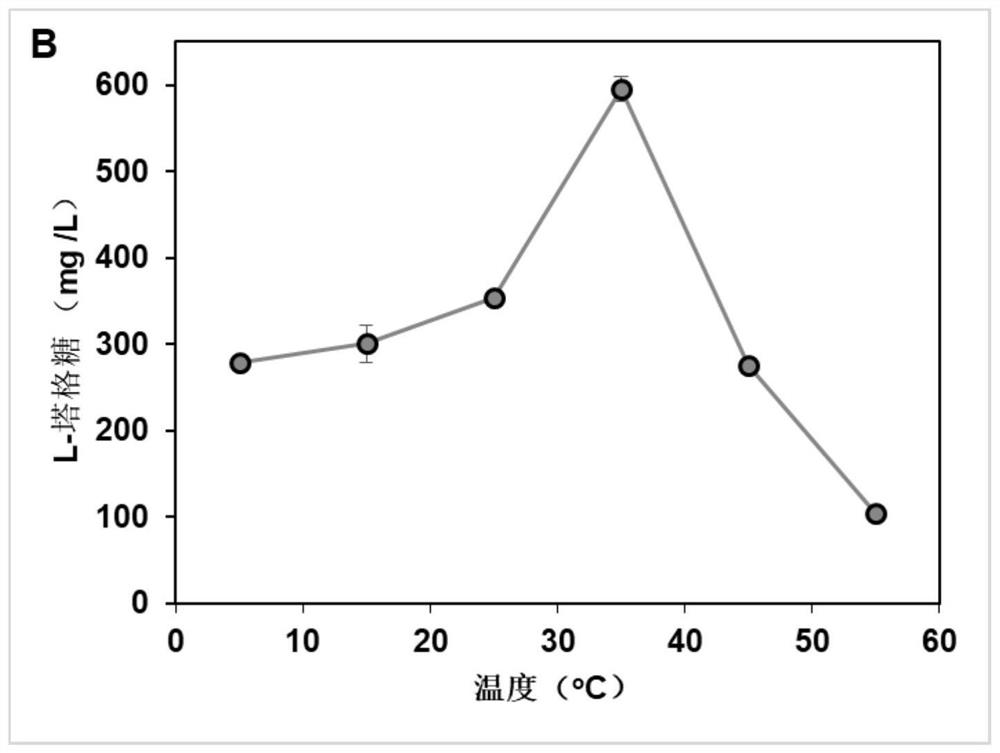
[0041] Weigh a certain amount of D-galactitol using pH 9.0 TRIS-HCl buffer to dissolve it, and configure it 100 mm. The reaction system is added to the neutralitol dehydrogenase liquid and NADH oxidase solution such that the final concentration of the latexal alcohol dehydrogenase in the reaction system is 1 U / mL, NADH oxidase liquid in the reaction system. The concentration was 2 U / mL, then 1 mm NAD + covic enzyme factor was added in the above reaction system, and its optimal reaction temperature was 35 ° C, the optimum reaction pH was 9.0, reacted at 26 h at magnetic stirring, and cysteine- Carbazole was measured to determine L-Taggosaccharide.
[0042] The L-Taggose is used to generate production and measurement methods as follows:
[0043] (1) Drawing of standard curves:
[0044] Preparation solution: For...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com