vzif-67/zif-67-polyimide mixed matrix film, preparation method and application thereof
A VZIF-67-, VZIF-67 technology, applied in the field of gas separation membranes, can solve the problems of staying in the experimental stage, no mass production, and the crystallization mechanism is not very perfect, so as to improve the separation performance, permeability and separation selection The effect of improving performance and eliminating interface defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of VZIF-67 nanoparticles of the present invention is as follows: 20 g of 2-MeIm and 1 mL of formaldehyde solution (mass concentration 37%) are added to 300 mL of distilled water and stirred for 15 min, which is designated as A solution. Then weigh 1 g of Co(NO 3 ) 6H 2 O. 0.1 g of 4-aminophenol was dissolved in a mixed solution of 150 mL of water and ethanol (water / ethanol=2 / 1), which was designated as solution B. Mix liquid A and liquid B, stir at room temperature for 12 h, and the resulting dark purple solid is collected by centrifugation, washed twice with water and ethanol, and dried at 60°C in a vacuum drying oven to obtain VZIF-67 nanoparticles.
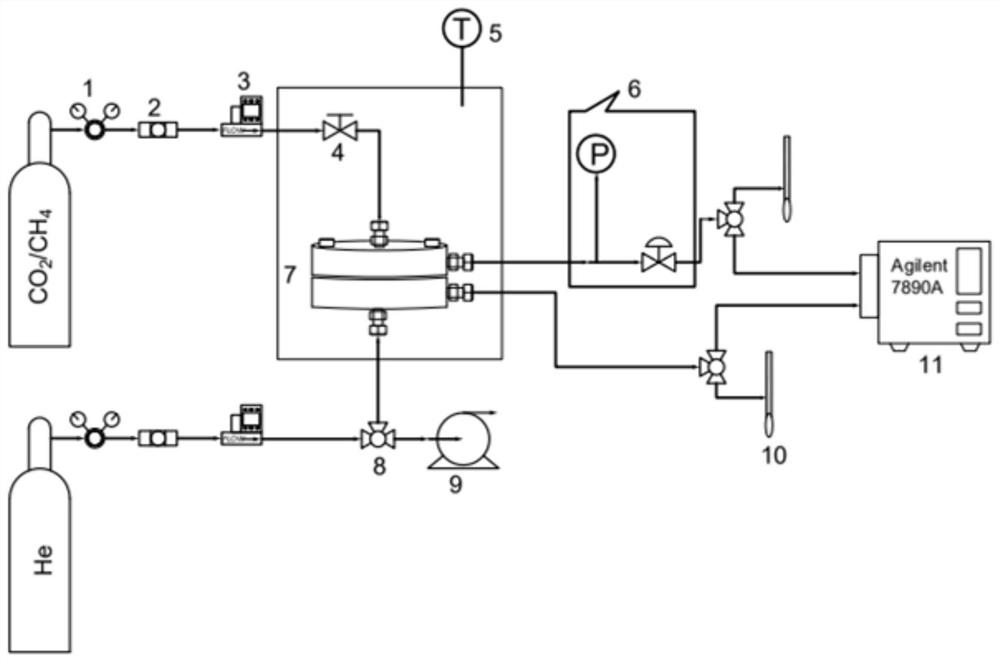
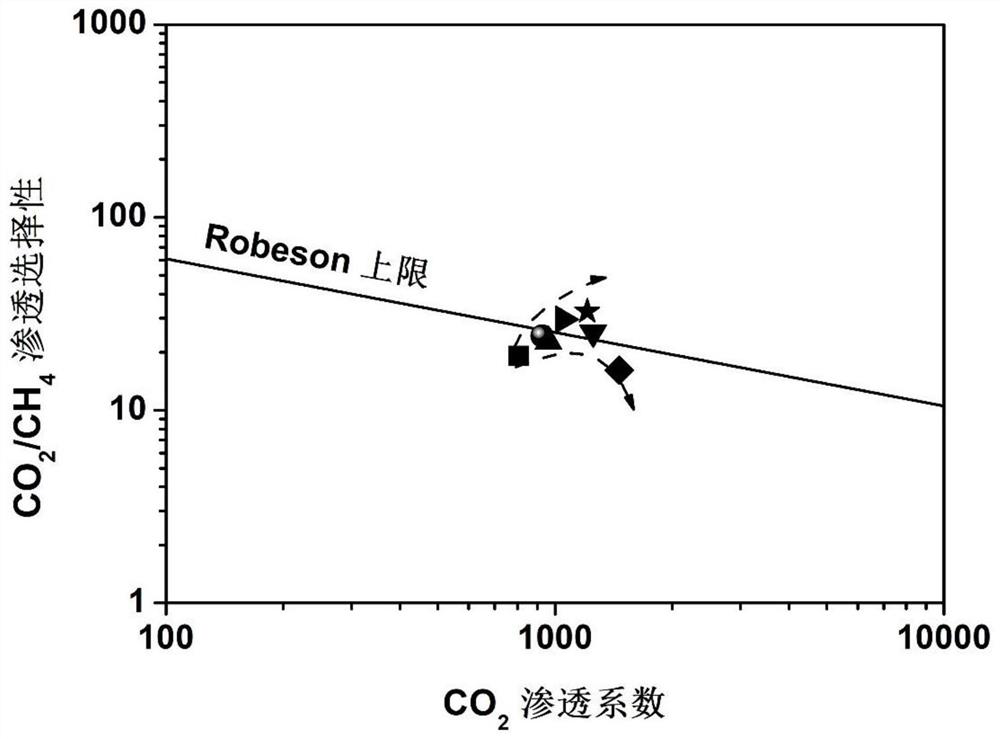
[0040] Example 1-2 and comparative example 1-2 permeability coefficient and selectivity test process is as follows: under 35 ℃, 2bar operating conditions, VZIF-67 (ZIF-67) / polyimide mixed matrix membrane permeability test ( The schematic diagram of the gas separation test device is shown in fi...
Embodiment 1
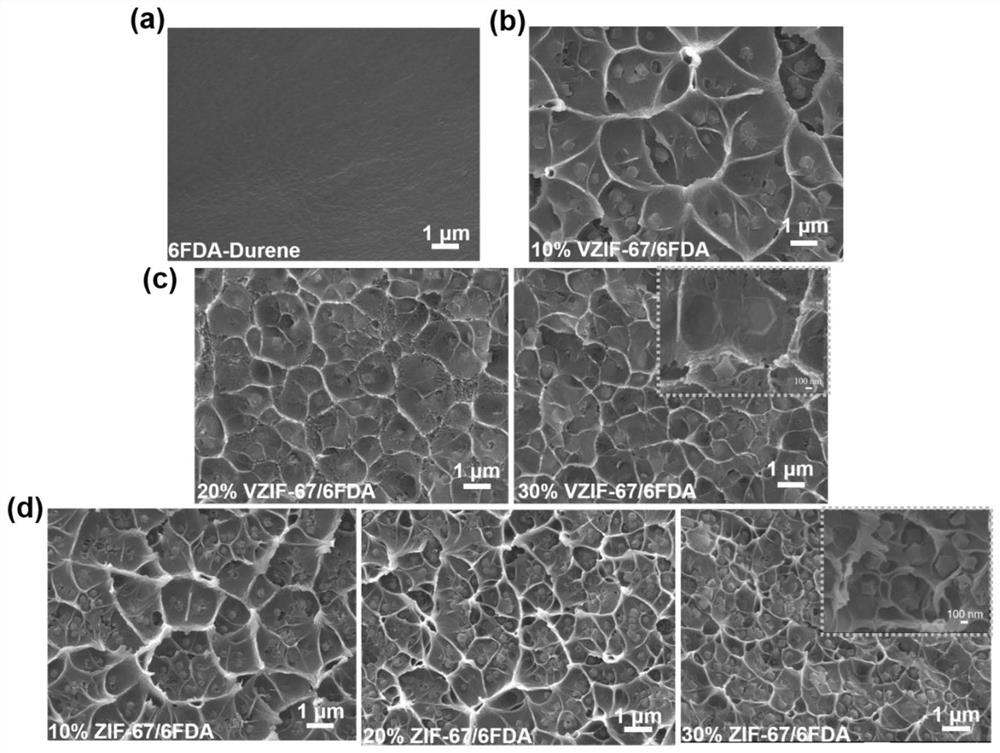
[0046] a) Accurately weigh 0.033 g of dried VZIF-67 nanoparticles into 4 mL of chloroform solution, ultrasonically disperse for 20 min, and then stir for 4 h, so that VZIF-67 nanoparticles are uniformly dispersed in the chloroform solution to form uniform VZIF-67 chloroform solution;
[0047] b) Accurately weigh 0.3g of dry 6FDA-Durene powder, add 0.03g from the weighed 6FDA-Durene to the VZIF-67 chloroform solution in step a), stir for 2h until completely dissolved, then add the remaining 6FDA-Durene -Durene powder is completely added to the solvent, stirred for 12h until completely dissolved, to obtain a uniformly dissolved casting solution, and then ultrasonically defoamed;
[0048] c) Pour the defoamed casting liquid into a 6cm-diameter PTFE petri dish, place it horizontally, buckle it with a glass funnel on the top of the PTFE petri dish, and wait for the solvent to evaporate under indoor conditions Film formation, and then put the formed film together with the film tool...
Embodiment 2
[0054] a) Accurately weigh 0.075g and 0.1285g of dry VZIF-67 nanoparticles and add them to 2 dry and clean brown reagent bottles respectively, and then accurately pipette 4mL of chloroform solution into these 2 reagent bottles with a pipette gun. , ultrasonically dispersed for 15min, and then magnetically stirred for 4h until the VZIF-67 nanoparticles were uniformly dispersed in the chloroform solution to form a uniform VZIF-67 nanoparticle solution;
[0055] b) Accurately weigh 2 parts of 0.3g dried 6FDA-Durene powder, respectively add one tenth of each part to the VZIF-67 nanoparticle solution in step a), stir until completely dissolved, and then add The remaining 6FDA-Durene powders were respectively added to the respective reagent bottles, stirred for 12h to be completely dissolved, and finally obtained the casting liquid with VZIF-67 nanoparticle content of 20wt.% and 30wt.%;
[0056] c) Pour the defoamed casting solution into a 6cm-diameter PTFE petri dish, place it hori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com