Application of mass spectrometry to detect the level of psm-e molecules in urine in the preparation of products for early diagnosis of prostate cancer
A technology for prostate cancer and urine, applied in the field of clinical examination and diagnosis, achieves the effects of wide application, convenient sampling and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of standard product of PSM-E characteristic peptide and preparation of standard working solution
[0035] 1. Experimental method
[0036] 1. Synthesis of PSM-E characteristic peptide standard
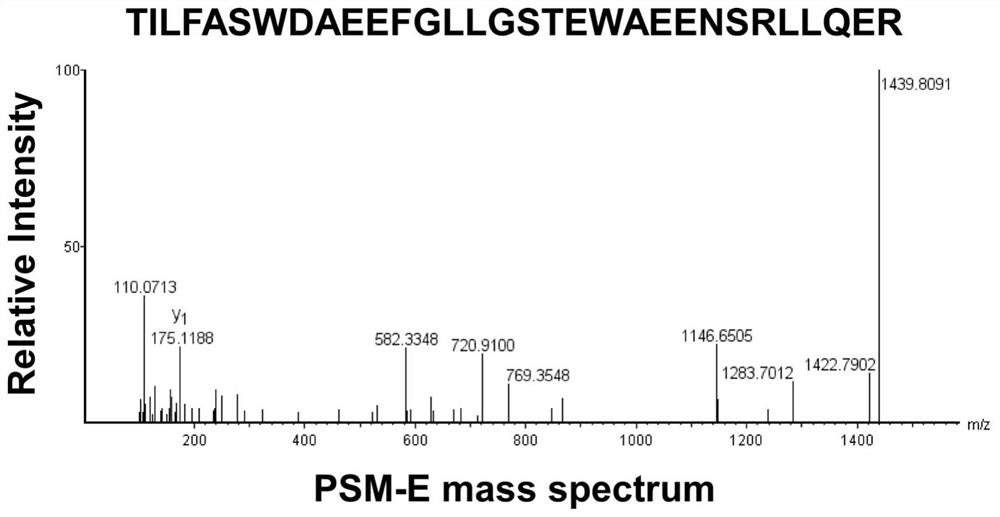
[0037] The standard PSM-E characteristic peptide was synthesized by Hangzhou Zhongpeptide Biochemical Co., Ltd., and its amino acid sequence is: TILFASWDAEEFGLLGSTEWAEENSRLLQER (SEQ ID NO: 2); 5 mg; purity 99.5%.
[0038] 2. Preparation of PSM-E Characteristic Peptide Standard
[0039] with ddH 2 O Dilute the BSA standard to 1 ng / μl. Set up 8 concentration gradients of protein labeling products: add the standard products to the standard product wells of the 96-well culture plate according to 0, 1, 2, 4, 8, 10, 12, 14, 16, 18, and 20 μl, less than 20 μl The wells were made up to 20 μl with ultrapure water.
[0040] Table 1 Standard sample addition table
[0041]
Embodiment 2
[0042] The detection of PSM-E characteristic peptide content in embodiment 2 urine
[0043] 1. Proteolysis in urine samples
[0044] The method is as follows:
[0045] (1) Transfer the urine sample to a corresponding 10KDa ultrafiltration tube, and centrifuge at 12,000g for 20min;
[0046] (2) Add 200μl of Buffer1 (8M urea / 100mM Tris-HCl, pH 8.5) to each ultrafiltration tube to fully dissolve the denatured protein;
[0047] (3) Add 20 μl DTT (100 mM) solution, react at 37°C for 2 h to reduce disulfide bonds;
[0048] (4) Add 20 μl of IAA (500 mM) solution, and react at room temperature for 15 min in the dark;
[0049] (5) Centrifuge the protein solution after reductive alkylation at 12,000 g for 20 min, and discard the solution at the bottom of the collection tube. Add 200uL Buffer1 and centrifuge, repeat twice;
[0050] (6) Add 200μl Buffer2 (8M urea / 100mM Tris-HCl, pH 8.0), centrifuge at 12,000g for 20min, discard the solution at the bottom of the collection tube, repea...
Embodiment 3
[0065] The detection of PSM-E characteristic peptide content in embodiment 3 urine
[0066] 1. Experimental method
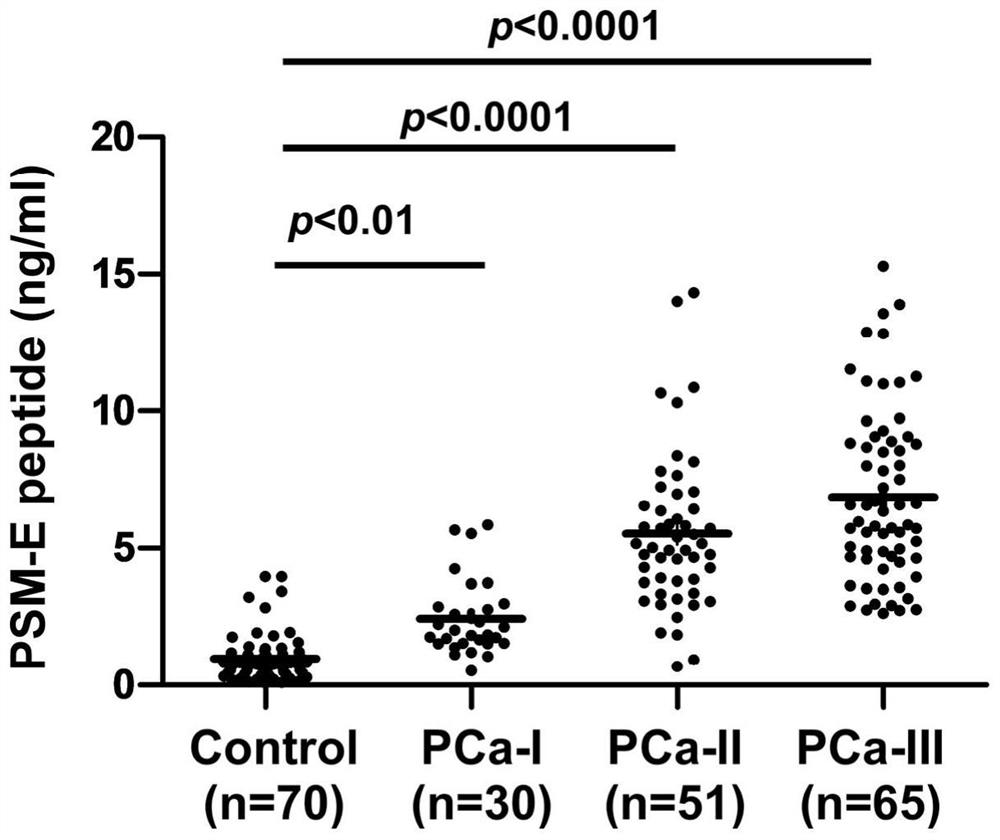
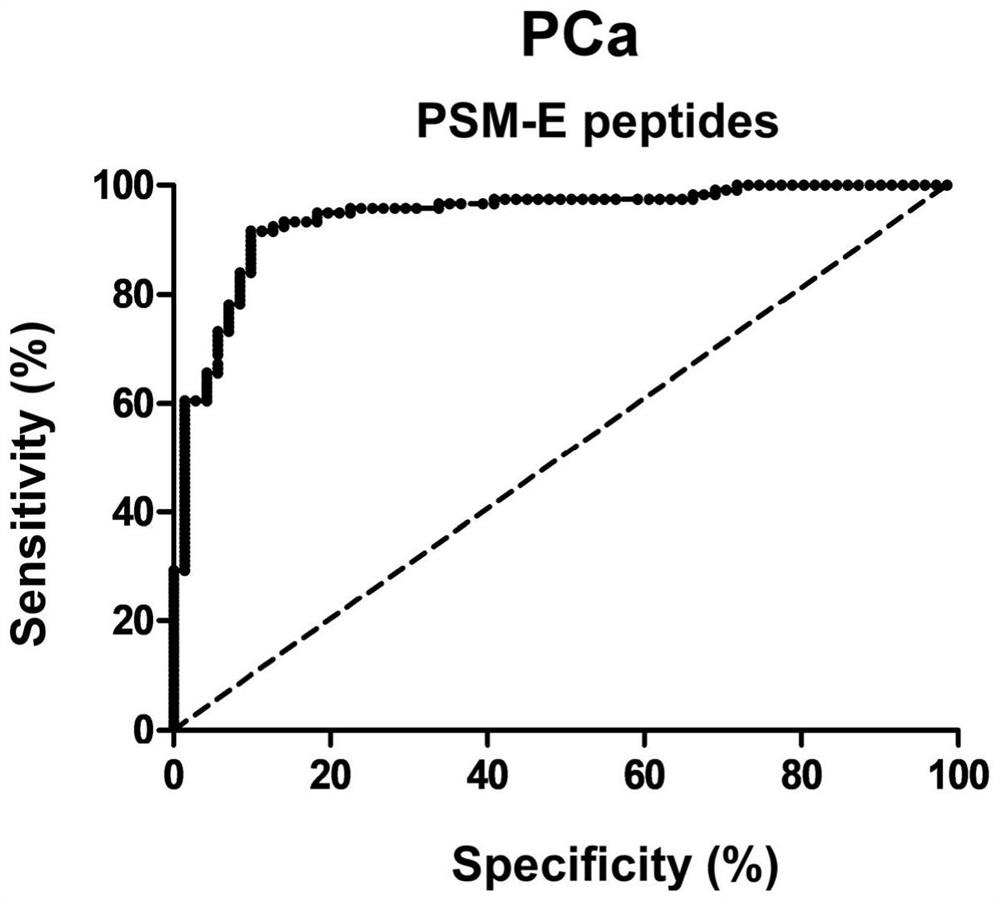
[0067] A total of 132 research subjects were selected, and there were no statistically significant differences in gender, age, blood pressure, smoking, and drug use among the groups, which were comparable. Data were collected from 70 healthy subjects (Control), 30 patients with stage I prostate cancer (PCa-I), 51 patients with stage II prostate cancer (PCa-II), and 65 patients with stage III prostate cancer (PCa-III). Serum, using the method of Example 3, to detect the content of PSM-E characteristic peptide in the urine samples of each group.
[0068] 2. Experimental results
[0069] like figure 2 and 3 As shown, the concentrations of PSM-E characteristic peptide (SEQ ID NO: 2) in the urine of the above groups were 0.9831±0.8322ng / ml, 2.4430±1.3780ng / ml, 5.5170±2.836ng / ml, 6.8740±3.1350, respectively ng / ml. Among them, compared with the healthy group, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com