Construction and application of near-infrared light activated macrophage-nano prodrug targeted drug delivery system
A technology of macrophages and near-infrared light, which is applied in nano-medicine, nano-technology, nano-optics, etc., can solve the problem of synchronous release of photosensitive molecules and cytotoxic chemotherapy drugs, synchronous delivery of photosensitive active molecules and synchronous delivery of macrophages. Chemotherapy prodrugs, immunogenic cell death and other problems, to achieve the effect of efficient drug delivery, high drug loading, and efficient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0062] Example 1: Preparation of light-activated prodrug nanocarriers:
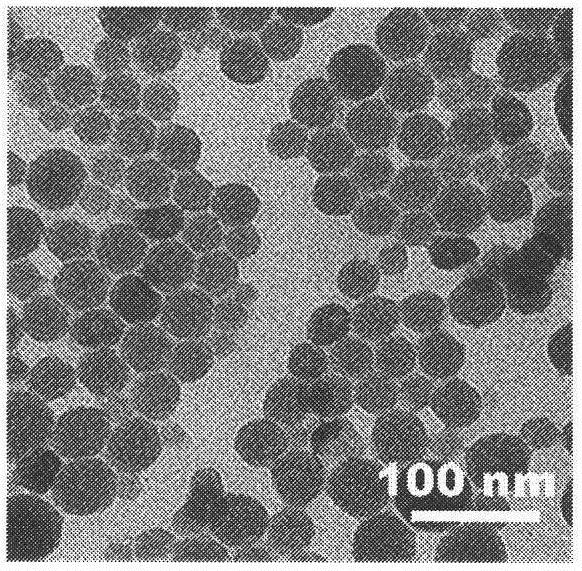
[0063] The DOPA-wrapped prodrug nanocarrier core was prepared by reverse microemulsion method. The specific operation is as follows: a cyclohexane solution containing 0.3M triton and 1.5M n-hexanol is prepared as the oil phase of the microemulsion method. Slowly add 200 μL carboxylated oxaliplatin sodium salt solution (8.83 mg / mL) into 5 mL oil phase as the drug phase, and simultaneously add 200 μL Zn 2+ The aqueous solution (25mg / mL) was slowly added to another same 5mL oil phase as the ionic phase, and each was stirred at room temperature for 5min to form a transparent W / O microemulsion. 30 μL of DOPA chloroform solution (138 mM) was added into the ionic phase, and after stirring for 5 min, the drug phase was slowly added dropwise into the ionic phase while stirring. After the mixed solution was continuously stirred at room temperature for 45 min, 10 mL of absolute ethanol was added, and the product...
example 2
[0066] Example 2: Acquisition of BMDM:
[0067] Firstly, L929 cell conditioned medium (L929-CM) is prepared, and the specific preparation operation is as follows: after L929 cells are subcultured, when the density reaches 50%-80%, continue to culture for 5-7 days. The supernatant was collected, centrifuged at 1000rpm for 6min, filtered through a 0.22μm filter membrane, dispensed into 5mL EP tubes, and stored in a -20°C refrigerator for later use.
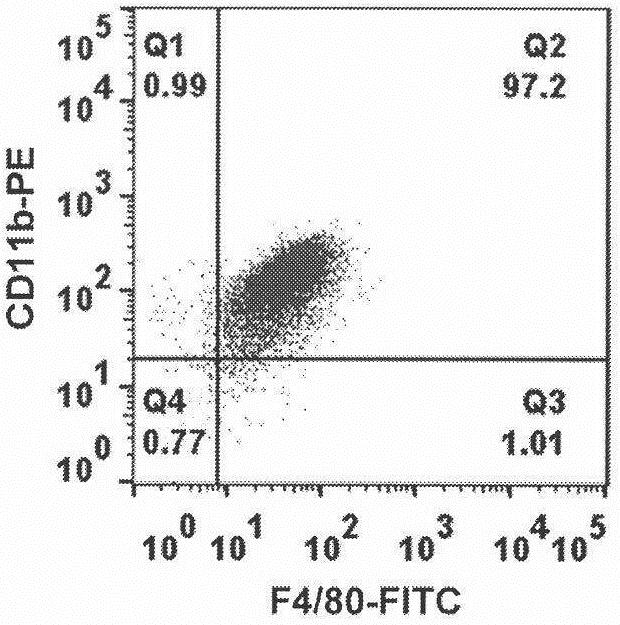
[0068] The preparation of BMDM is as follows: C57BL / 6 mice aged 8-12 weeks were sacrificed by cervical dislocation and immediately soaked in 75% ethanol solution for 5 minutes, then transferred to the ultra-clean bench, and the skin and muscles were quickly separated by reverse dissection, free and clean and intact femur and tibia. After washing with sterile pre-cooled PBS, the bone marrow cavity was carefully exposed with surgical scissors, and a 10 mL syringe with a 1 mL syringe needle was used to absorb serum-free DMEM and ins...
example 3
[0070] Example 3: Cell Carrier Drug Loading Determination:
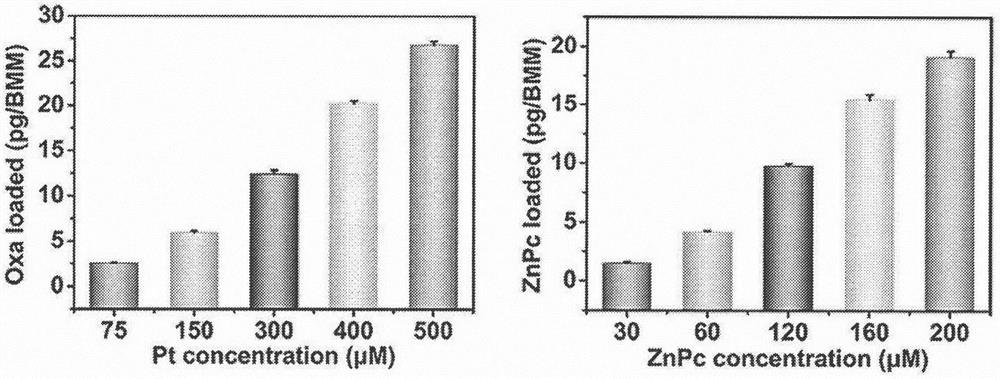
[0071] The same number of BMDMs (1×10 6 1) were incubated with the same volume of different concentrations of prodrug nanocarriers at 37°C for 2 h, wherein the incubation concentrations of platinum were 500, 400, 300, 150 and 75 μM; the corresponding incubation concentrations of photosensitizers were 200, 160, 120 , 60 and 30 μM. After incubation, the cells were washed 3 times with PBS. The content of photosensitizer zinc phthalocyanine in the cells was measured by fluorescence spectrophotometer. The specific method is: after the incubation, the cells were washed 3 times with PBS, the cells were collected and lysed with cell lysate, the aqueous solution was removed by rotary evaporation at 50°C, and then dissolved by adding chloroform to destroy lipid molecules such as lipid cell membranes, DOPC and cholesterol For the coating of zinc phthalocyanine, free zinc phthalocyanine is released, then chloroform is remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com