Single-domain antibody targeting human IgE, humanized single-domain antibody and application thereof
A single-domain antibody and humanized technology, which is applied in applications, solid adsorbent liquid separation, and other chemical processes, can solve the problems of poor protein stability, difficulty in long-term storage, increased complexity of clinical treatment, and difficulty in obtaining ligands. , to achieve the effect of low immunogenicity, small molecular weight and good regenerative performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Prokaryotic expression and purification of single domain antibody
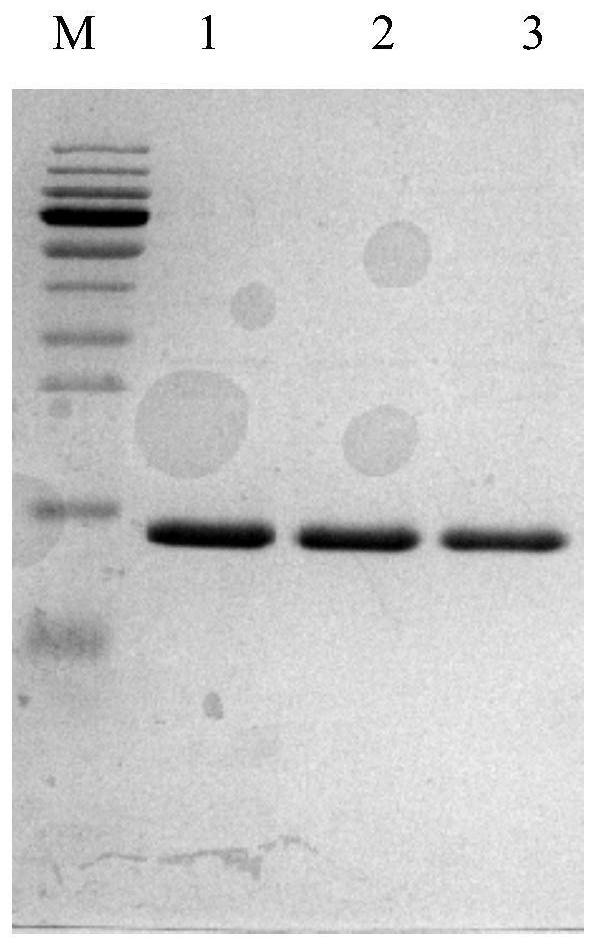
[0080] The construction and screening methods of the phage library are the same as CN111875706A, and the positive clones obtained by screening are sequenced. The nucleotide sequences shown in SEQ ID NO.: 5, SEQ ID NO.: 10 and SEQ ID NO.: 12 were inserted into the pet-28a plasmid, and the DNA restriction sites were designed as NcoI and XhoI. Then the plasmid was transformed into Escherichia coli BL21 (DE3), cultured in kanamycin-resistant LB medium, induced by 1 mM IPTG for 4 hours, the broken bacteria were centrifuged to take the supernatant, and the anti-IgE single was adsorbed by cobalt ion packing. Domain antibodies such as figure 1 shown. from figure 1 It can be seen that the molecular weight of the single domain antibody is about 14KD, which is in line with the theoretical value.
Embodiment 2
[0081] Embodiment 2 Anti-IgE single domain antibody activity comparison
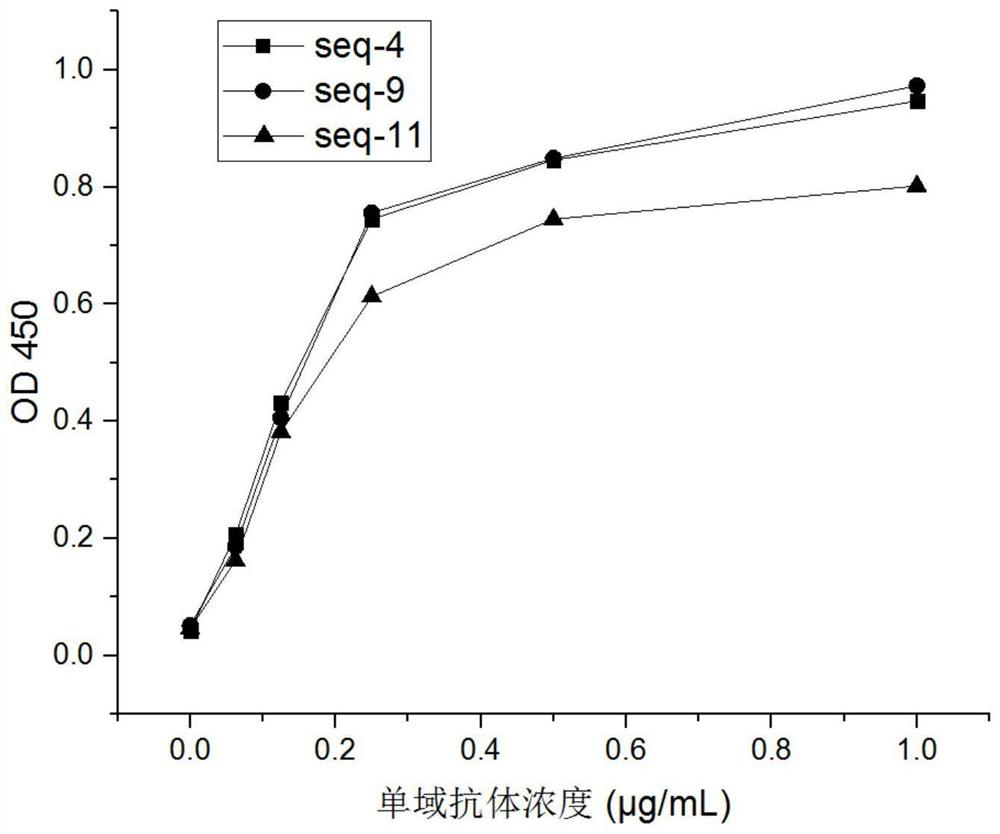
[0082] Dilute the natural human IgE protein (abcam) to 1ug / ml and coat it on the microtiter plate, dilute the prokaryotic-expressed single-domain antibodies with a concentration of 1ug / ml to a certain concentration, add to the microtiter plate, react at room temperature for 2 hours, and then wash Plate 5 times, add HRP-labeled anti-His antibody, react at room temperature for 1 hour, then add TMB chromogenic solution and stop solution, measure OD 450 value. The result is as follows figure 2 shown. The results showed that the activity of the alpaca single domain antibody shown in SEQ ID NO.: 4 and SEQ ID NO.: 9 was not much different, and the activity of the humanized single domain antibody decreased by about 13%, which was within an acceptable range .
Embodiment 3
[0083] Embodiment 3 synthetic adsorption packing
[0084] (1) Take 5mL of agarose gel and add 2M NaOH solution at a volume ratio of 1:1, and add an activator (glycerol ether / epichlorohydrin) at a volume ratio of 1:0.7 to react to obtain a gel with a large number of epoxy groups on the surface. Agarose gel;
[0085] (2) Wash the epoxidized agarose with PBS, add 0.5g of 6-aminocaproic acid, and react in a shaker at 37°C and 180rpm for 2h, the epoxy groups on the surface of the agarose and the 6-aminocaproic acid Amino groups are reacted to obtain agarose gel with carboxyl groups on the surface;
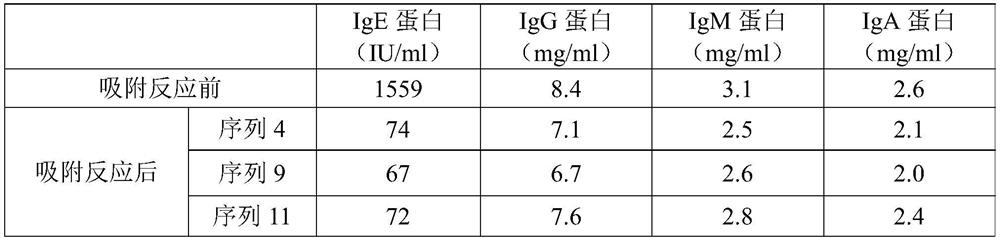
[0086] (3) After the carboxylated agarose was washed with PBS, 0.5 g of EDC / NHS was added, and at the same time, 100 mg of each antibody solution (SEQ ID NO.: 4, SEQ ID NO.: 9, SEQ ID NO.: 11) was reacted, 30°C, 120rpm mixing and shaking for 14h, after the synthesis was completed, it was washed with PBS and set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com