Chiral beta-diester selenide compound, and preparation method thereof through Michael addition
A compound and diester technology, which is applied in the field of preparation of chiral beta-diester selenide compounds and their Michael addition, can solve the problems of low enantioselectivity, limited substrates and the like, and achieve simplified operation steps, High reaction efficiency, safe and controllable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
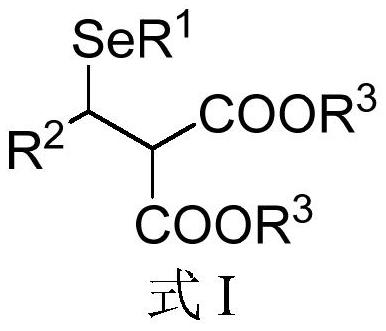
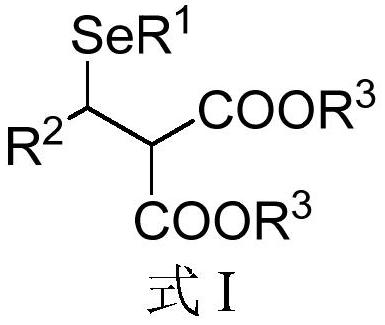
[0070] The second aspect of the embodiment of the present application provides a preparation method of Michael addition of chiral β-diester selenide compounds, comprising the following steps:
[0071] S01. Selenol compound A and ene diester compound B shown in the general structural formula are provided:
[0072] A:R 1 -SeH B:
[0073] S02. Adding the selenol compound A and the olefin diester compound B into a reaction system containing a nitrogen heterocyclic carbene catalyst, an alkaline reagent and a water-absorbing additive, and reacting at a temperature of -100°C to 25°C, A chiral β-diester selenide compound represented by formula I is obtained.
[0074] Specifically, in the above step S01, R in the molecular structural formula of selenol compound A 1 The represented group is the same as R in the general formula I of the molecular structure of the above chiral β-diester selenide compound 1 The groups represented are the same. R in the molecular structural formula o...
Embodiment 1
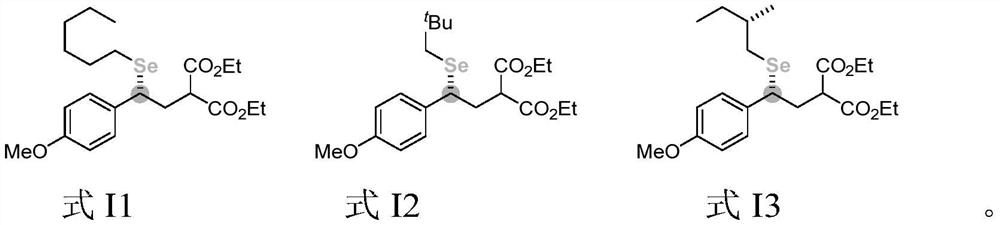
[0094] This example provides a diethyl (R)-2-(2-(hexylselenyl)-2-(4-methoxyphenyl)ethyl)malonate and a preparation method thereof. The structural formula of the ((R)-2-(2-(hexylselenyl)-2-(4-methoxyphenyl)ethyl)diethyl malonate is shown in molecular structural formula I1 below:
[0095]
[0096] Its preparation steps are as follows:
[0097] In a 20ml test tube, dissolve triazolecarbene-thiourea bifunctional catalyst (0.01mmol, 0.1 equivalent (equiv.)), 13X molecular sieve (100mg) and n-hexylselenol nucleophile (0.2mmol, 2.0equiv.), In a mixed solvent of 1.2 mL of pretreated diethyl ether, seal it with a rubber stopper, then replace the gas (3 times) under an argon atmosphere, and slowly add lithium bis(trimethylsilyl)amide (LiHMDS) (1mol / L, tetrahydrofuran / ethylbenzene solution, 10 μL, 0.10 equiv.), the gas was replaced again under argon atmosphere (3 times). The tube was sealed with Parafilm and then stirred at -90°C for 1 hour. Diethyl (E)-2-(4-methoxystyryl)malonate...
Embodiment 2
[0100]This example provides a diethyl (R)-2-(2-(4-methoxyphenyl)-2-(neopentylselenoyl)ethyl)malonate and a preparation method thereof. The structural formula of the (R)-2-(2-(4-methoxyphenyl)-2-(neopentylselenoyl) ethyl) malonate diethyl malonate is shown in molecular structural formula I2 below:
[0101]
[0102] Its preparation method refers to the preparation method of (R)-2-(2-(hexylselenyl)-2-(4-methoxyphenyl)ethyl)diethyl malonate in Example 1, the difference is Neopentylselenol (0.2 mmol) was used instead of n-hexylselenol. The reaction solution was directly separated and purified by silica gel column chromatography (ethyl acetate and n-hexane as eluents) to obtain the target product, a colorless liquid, with a yield of 96% and an ee value of 95%.
[0103] The prepared product I2 is subjected to characterization data analysis, and the result is 1 H NMR (400MHz, CD 2 Cl 2 )δ7.23(d, J=8.6Hz, 2H), 6.83(d, J=8.6Hz, 2H), 4.45(d, J=11.7Hz, 1H), 4.30-4.17(m, 2H), 4.03- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com