Method for preparing two alkalis
A solution and compound technology, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of easy oxidation, high environmental protection pressure, high operation risk, etc., and achieve the effect of easy industrial scale-up, realizing recycling, and avoiding oxidation deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
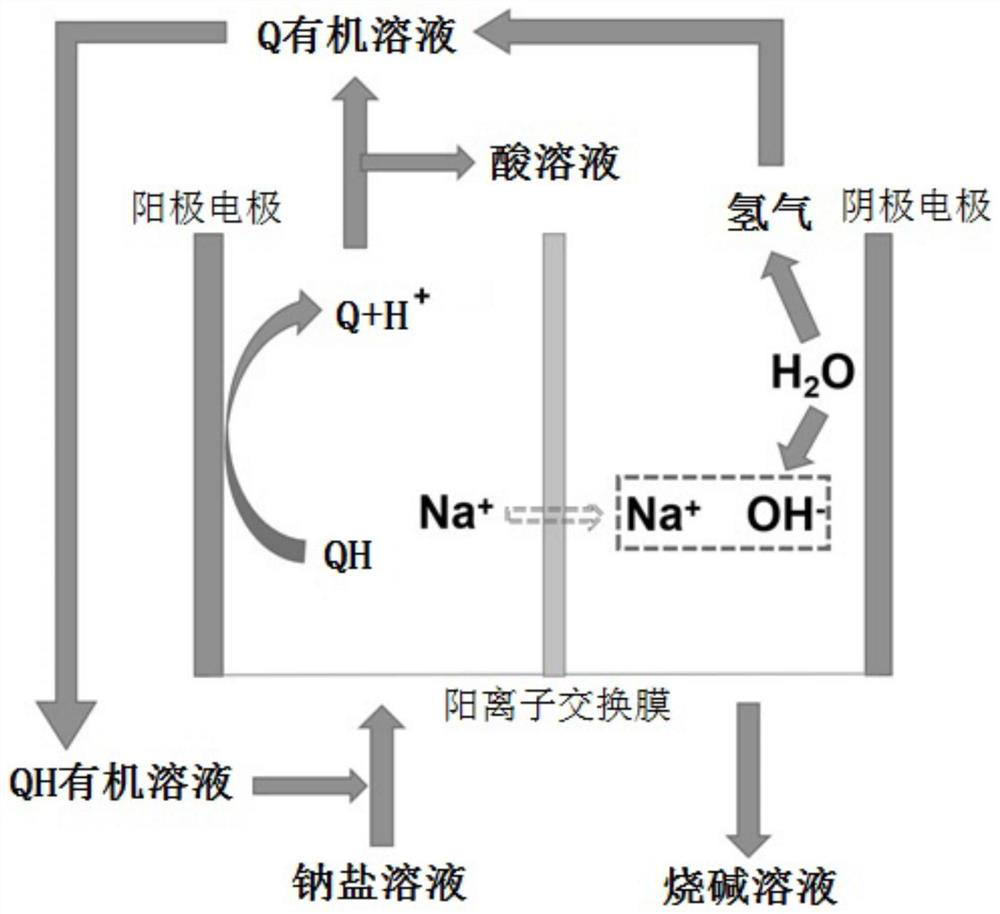
[0038] Embodiment 1, a method for preparing caustic soda, the electrolytic cell is divided into an anode area and a cathode area by using a perfluorosulfonic acid cation exchange membrane with a carboxylic acid layer. Hydrogen evolution electrode.
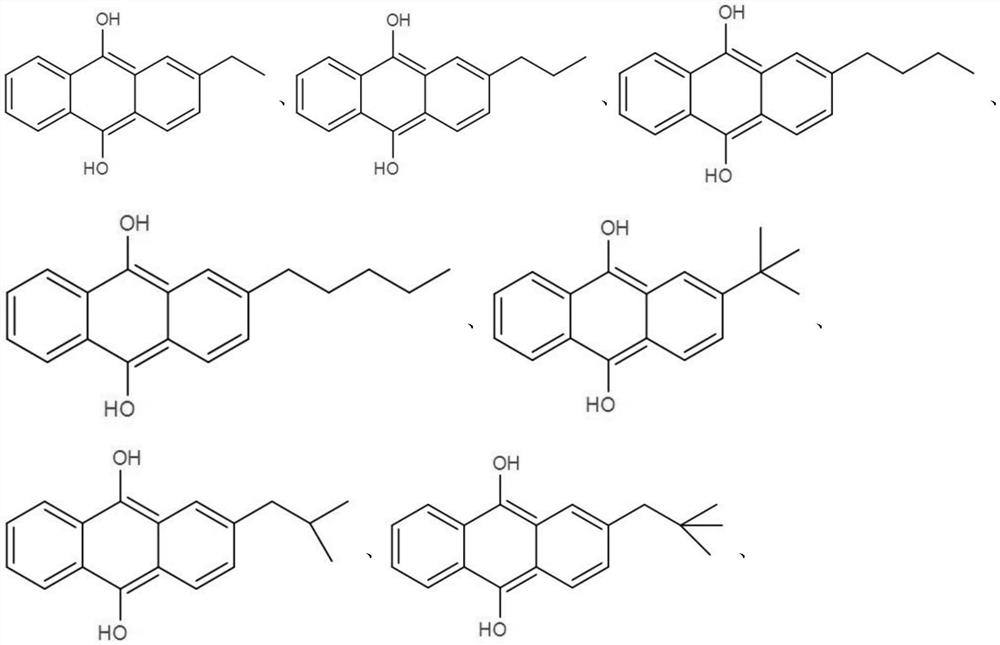
[0039] The structural formula is The compound QH of the compound QH is used as an anode electrocatalyst. The compound QH is dissolved in the mixed solution of 1,2 dichloroethane and n-butanol to obtain an organic solution with a compound QH concentration of 0.1mol / L, and the concentration of 0.3 is added to the organic solution mol / L 1-butyl-3-methylimidazolium hexafluorosulfate ionic liquid was used as a supporting electrolyte. A sodium acetate solution with a concentration of 2 mol / L is configured as a sodium salt solution, which is circulated into the anode area of the electrolytic cell together with the organic solution dissolved in the compound QH. The cathode area is configured with 30wt.% sodium hydroxide solution as ca...
Embodiment 2
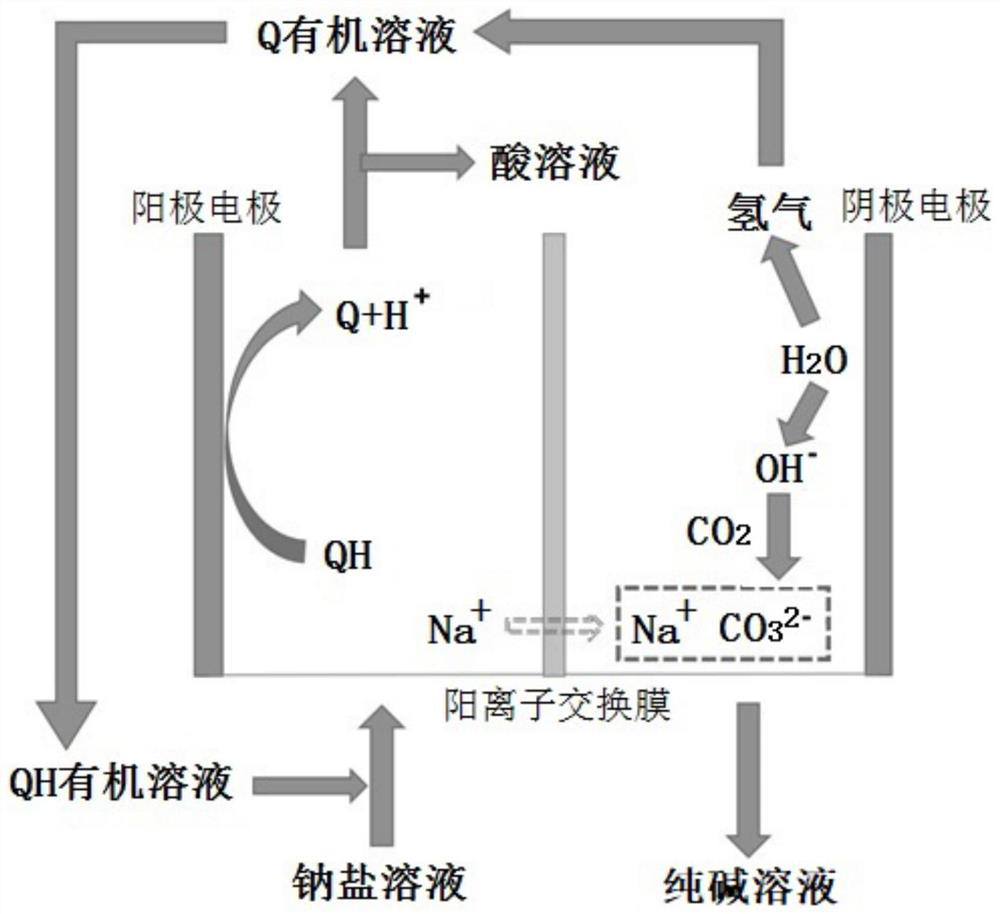
[0052] Embodiment 2, a method for producing soda ash. The electrolytic cell is divided into an anode area and a cathode area by using a cation exchange membrane. The anode electrode is made of carbon fiber cloth, and the cathode electrode is made of a nickel-plated platinum mesh as the hydrogen evolution electrode.
[0053] The structural formula is The compound QH of the compound QH is used as an anode electrocatalyst. The compound QH is dissolved in the mixed solution of 1,2 dichloroethane and n-butanol to obtain an organic solution with a compound QH concentration of 0.1mol / L, and the concentration of 0.3 is added to the organic solution mol / L 1-butyl-3-methylimidazolium hexafluorosulfate ionic liquid was used as a supporting electrolyte. A sodium acetate solution with a concentration of 2 mol / L is configured as a sodium salt solution, which is circulated into the anode area of the electrolytic cell together with the organic solution dissolved in the compound QH. The ca...
Embodiment 3
[0062] Embodiment 3, a method for producing soda ash. The electrolytic cell is divided into an anode area and a cathode area by using a cation exchange membrane. The anode electrode is made of carbon fiber cloth, and the cathode electrode is made of a nickel-plated platinum mesh as a hydrogen evolution electrode.
[0063] The structural formula is The compound QH of the compound QH is used as an anode electrocatalyst. The compound QH is dissolved in the mixed solution of 1,2 dichloroethane and n-butanol to obtain an organic solution with a compound QH concentration of 0.1mol / L, and the concentration of 0.3 is added to the organic solution mol / L 1-butyl-3-methylimidazolium hexafluorosulfate ionic liquid was used as a supporting electrolyte. A sodium acetate solution with a concentration of 2 mol / L is configured as a sodium salt solution, which is circulated into the anode area of the electrolytic cell together with the organic solution dissolved in the compound QH. The catho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com