Ibuprofen quick-release and slow-release nanoparticle and preparation method thereof
A nanoparticle, slow-release technology, applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of inability to exert drug efficacy for a long time, slow initial release, loss of drug efficacy, etc., to achieve continuous maintenance of blood drug concentration, Quick release, prolonged storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
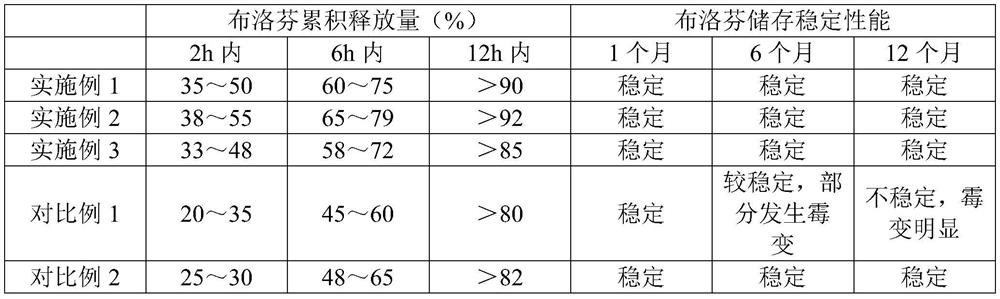
Examples
Embodiment 1
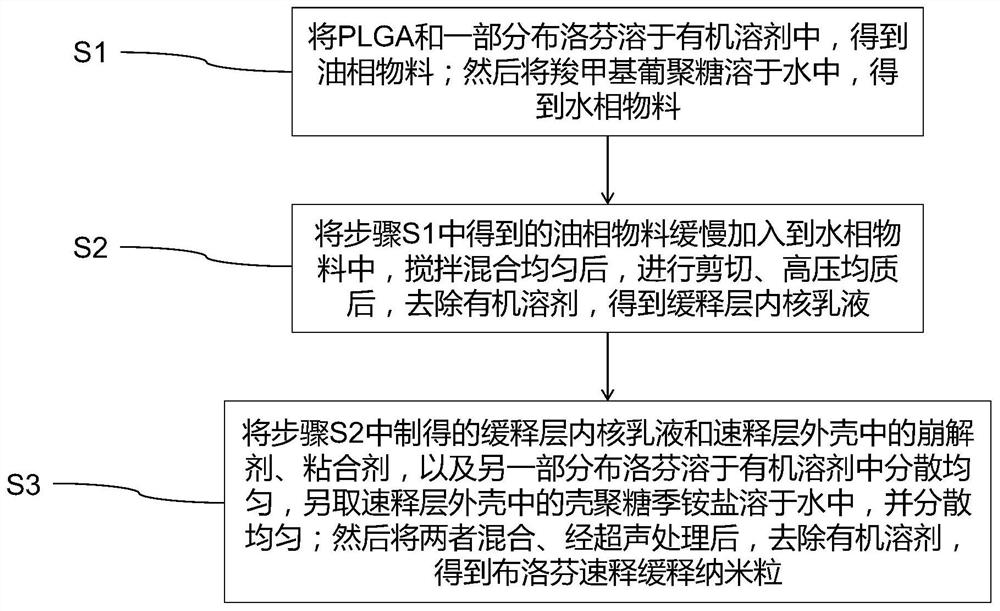
[0031] The preparation method of described ibuprofen quick-release sustained-release nanoparticles, comprises the steps:
[0032] S1. Dissolving PLGA and a part of ibuprofen in ethanol to obtain an oil phase material; then dissolving carboxymethyl dextran in water, the PLGA copolymer, the carboxymethyl dextran and the part of cloth The mass ratio of profen is 15:3:0.5 to obtain the water phase material;
[0033] S2. Slowly add the oil phase material obtained in step S1 into the water phase material, stir and mix evenly, and then perform shearing and high-pressure homogenization. The shearing conditions are as follows: shear emulsification at 10,000 rpm for 25 minutes The conditions of the high-pressure homogenization are as follows: the homogenization temperature is 20°C, the homogenization pressure is 130 par, and the homogenization is performed twice; the organic solvent is removed to obtain the inner core emulsion of the slow-release layer;
[0034]S3, dissolving the crosc...
Embodiment 2
[0036] The preparation method of described ibuprofen quick-release sustained-release nanoparticles, comprises the steps:
[0037] S1. Dissolve PLGA and a part of ibuprofen in chloroform and ether to obtain an oil phase material; then dissolve carboxymethyl dextran in water, the PLGA copolymer, the carboxymethyl dextran and the The mass ratio of a part of ibuprofen is 18:6:1.0 to obtain the water phase material;
[0038] S2. Slowly add the oil phase material obtained in step S1 into the water phase material, stir and mix evenly, and then perform shearing and high-pressure homogenization. The shearing conditions are as follows: shear emulsification at 12000rpm for 30min The conditions of the high-pressure homogenization are as follows: the homogenization temperature is 25°C, the homogenization pressure is 140 par, and the homogenization is performed 3 times; the organic solvent is removed to obtain the inner core emulsion of the slow-release layer;
[0039] S3, dissolving the s...
Embodiment 3
[0041] The preparation method of described ibuprofen quick-release sustained-release nanoparticles, comprises the steps:
[0042] S1. Dissolve PLGA and a part of ibuprofen in acetone to obtain an oil phase material; then dissolve carboxymethyl dextran in water, the PLGA copolymer, the carboxymethyl dextran and the part of cloth The mass ratio of profen is 20:8:1.5 to obtain the water phase material;
[0043] S2. Slowly add the oil phase material obtained in step S1 into the water phase material, stir and mix evenly, and then perform shearing and high-pressure homogenization. The shearing conditions are as follows: shear emulsification at 15,000 rpm for 35 minutes The conditions of the high-pressure homogenization are as follows: the homogenization temperature is 30°C, the homogenization pressure is 150 par, and the homogenization is performed 4 times; the organic solvent is removed to obtain the inner core emulsion of the slow-release layer;
[0044] S3, dissolving the crospo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com