Synthesis process method of avobenzone
A process method and avobenzone technology are applied in the field of preparing avobenzone, can solve the problems of separation and purification of avobenzone, the total yield is only 87-90.5%, etc. The effect of equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0081] Under the protection of nitrogen, add 98.0g of 30% potassium methylate methanol solution into a dry and clean four-necked flask, control the temperature of the kettle at 25-28°C, and drop 60.0g of liquid p-methoxyacetophenone into the methanol within 1h In the potassium solution, after the dropwise addition, the heat preservation reaction was continued for 30 minutes to obtain 158.0 g of potassium p-methoxystyrene alcohol salt solution, which was transferred to the condensation process for later use.
[0082] Under nitrogen protection, add 600.0g o-xylene and 80.0g methyl p-tert-butylbenzoate into a 2000mL four-neck flask equipped with a stirrer, thermometer and decompression reflux water separation device, and the water pump controls the vacuum degree in the kettle to 18 ~25mmHg, the temperature of the kettle is controlled at 80~85°C, add 158.0g of p-methoxystyrene alcohol potassium salt solution dropwise, and methanol is extracted at the same time. The time of dropping...
experiment example 1
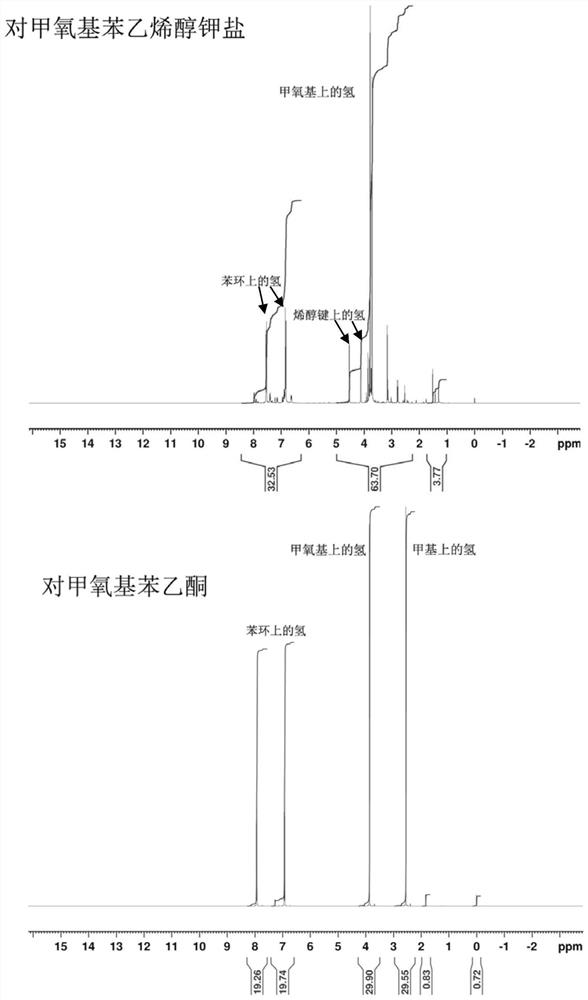
[0098] Get the p-methoxystyrene alcohol potassium salt that obtains in embodiment 1 and carry out nuclear magnetic resonance spectrum analysis, use INOVA400MHz high-resolution nuclear magnetic resonance spectrometer, TMS is internal standard, CDCl 3 for the solvent. The NMR spectra of p-methoxyacetophenone and potassium salt of p-methoxystyryl alcohol are as follows figure 1 shown.
[0099] from figure 1 As can be seen in the figure, after the reaction finishes, the resonance peak δ(2.55, 3H) representing the hydrogen atom on the methyl group connected to the carbonyl in p-methoxyacetophenone almost disappears, and a representative p-methoxystyrene appears. Resonant peaks δ(4.15, H) and δ(4.60, H) of the hydrogen atom on the enol bond in potassium alkoxide. δ(3.76, H) is the hydrogen on the methoxy group, and δ(6.92, 2H) and δ(7.65, 2H) are the hydrogens on the benzene ring, indicating that the reaction has yielded potassium p-methoxystyrene alkoxide.
experiment example 2
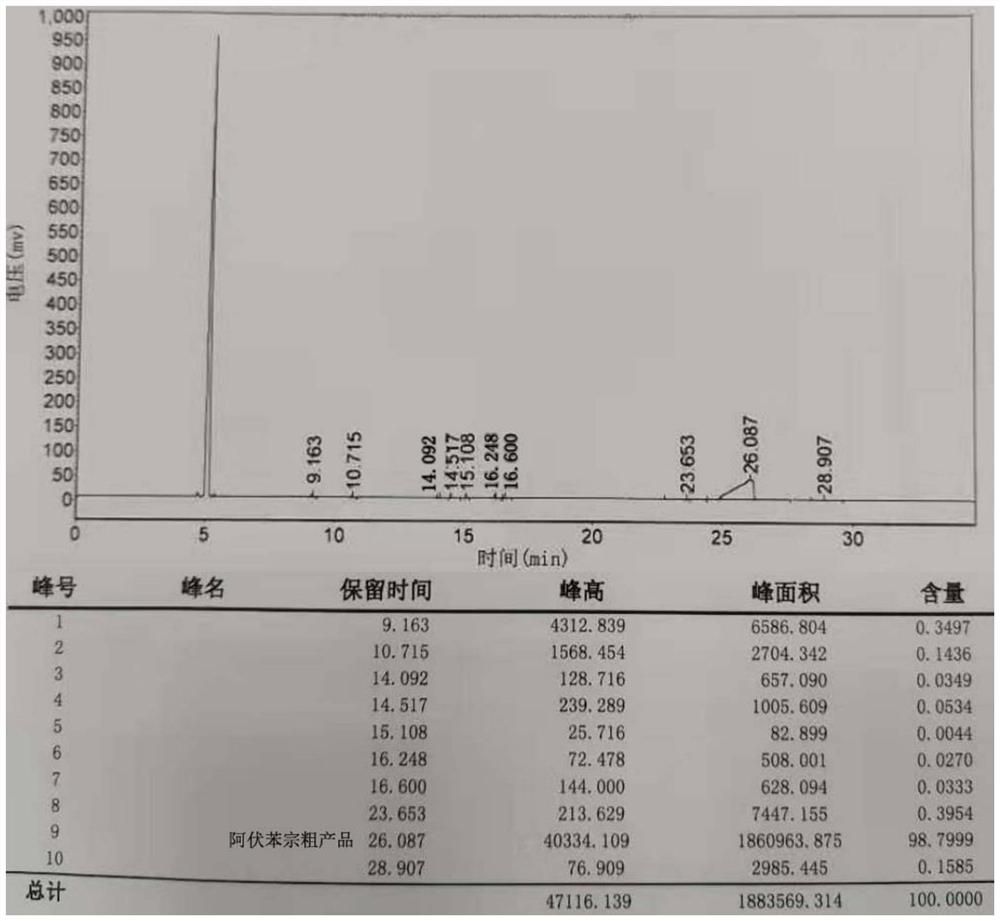
[0101] The crude product of avobenzone obtained after hydrolysis, neutralization and separation of the organic phase in Example 1 was analyzed by gas chromatography. The chromatographic column for chromatographic analysis is HP-5, nitrogen is used as carrier gas, the detector is flame ionization detector (FID), and the detector temperature is 300°C. The column oven was kept at 60°C for 3 minutes, then raised to 280°C at a rate of 30°C / min, and kept for 10 minutes. Chromatograms such as figure 2 shown. Wherein, the 26.087min place corresponds to the retention time of avobenzone, and the 5min place is the solvent o-xylene for dissolving the sample, the solvent is not counted, and the purity of avobenzone in the crude product is 98.7999%, illustrating the process in the present invention Control can effectively improve the selectivity of the target product and greatly reduce the formation of by-products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com