Antibacterial peptide and application thereof in aquaculture
A technology of antimicrobial peptides and bacteria, applied in the field of antimicrobial peptides and their application in aquaculture, can solve the problems of limited natural extraction resources of antimicrobial peptides, high cost of chemically synthesized peptides, complicated separation and purification process, etc. The effect of drug properties, chemical synthesis difficulty, and broad-spectrum antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 antimicrobial peptide caNKL2 102-119 selection and synthesis
[0031] 1. Acquisition of NK-lysins sequence of crucian carp
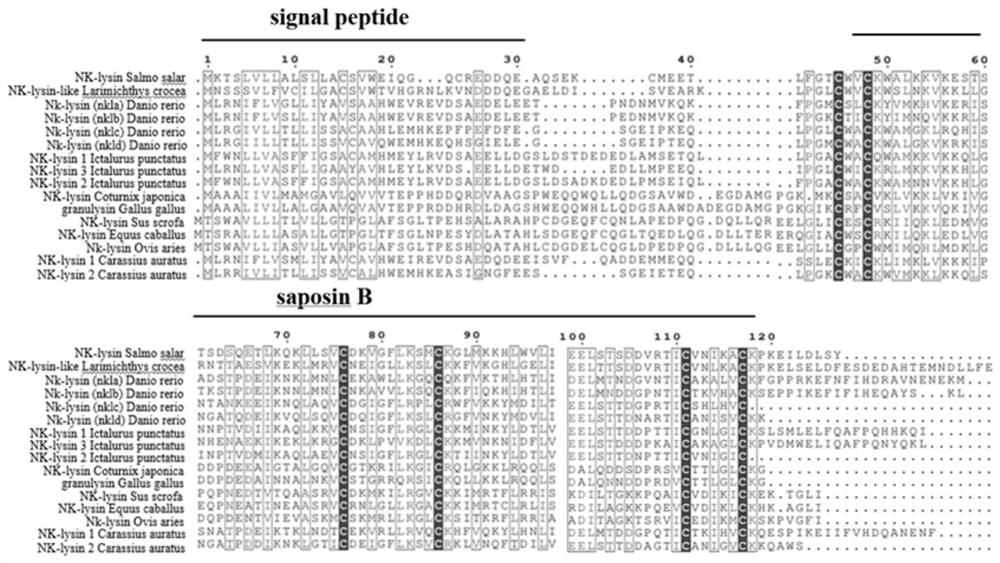
[0032] In order to find out the possible AMP gene in crucian carp, using the AMP gene sequences of zebrafish, grass carp, rainbow trout, carp, etc. (http: / / www.ncbi.nlm.nih.gov / blast) BLASTP search program analyzed the similarity between the homologous sequences of crucian carp AMP and other reported fish AMPs. ClustalX 2.0 was used to perform multiple sequence alignments on the searched AMP amino acid sequences, MEGA 5.0 software was used to construct a phylogenetic tree, and combined with PCR amplification and tissue quantitative data to confirm, two segments of crucian carp NK-lysins sequences were named caNK-lysins1 and caNK respectively. -lysins2, as a result of multiple sequence alignment, it was found that NK-lysins sequences all contain signal peptide, saposin B domain and 6 conserved cysteines (such as figure 1 and figur...
Embodiment 2
[0035] Embodiment 2 antimicrobial peptide caNKL2 102-119 Antibacterial activity verification
[0036] Testing the Synthetic Peptide caNKL2 Using the Oxford Cup Method 102-119 For the antibacterial activity against Escherichia coli, Staphylococcus aureus and Aeromonas hydrophila, kanamycin was used as a positive control, and the dissolution carrier was formic acid.
[0037] First, Escherichia coli, Staphylococcus aureus and Aeromonas hydrophila were streaked and inoculated on LB solid medium (Luria-Bertani medium), placed in a constant temperature incubator at 37°C for 18 hours, and single colonies of each strain were picked Placed in LB liquid medium, cultured at 37°C with constant temperature and shaking for 12h. Measure the OD of the bacterial solution 630 value (the absorbance value of the solution at a wavelength of 630nm), and dilute it to 1×10 6 CFU / mL. Then the bacteria were co-incubated with a series of antimicrobial peptide solutions with different final concentr...
Embodiment 3
[0040] Example 3 Antimicrobial Peptide caNKL2 102-119 cytotoxicity assay
[0041] In this example, the activity of the cells is detected by the MTT assay, and then the antimicrobial peptide caNKL2 is tested 102-119 Whether it is toxic to fish cells. The animal cells used in the experiment are carp epithelioma cell line (Epithelioma papulosum cyprini cell line, EPC).
[0042] Experimental principle: MTT entering living cells can be acted upon by intracellular enzymes to produce blue-purple crystals, which are deposited in the cells. Dead cells cannot form blue-purple crystals. The organic solvent DMSO can dissolve the blue-purple precipitate formed. Use a spectrophotometer to detect the absorbance of the blue-purple crystals in the sample, which can reflect the activity of the cells. The absorbance value of blue-purple crystals in the sample is positively correlated with the number of living cells.
[0043] Specific steps are as follows:
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com