Plasmid vector capable of simultaneously expressing PLAUR and GPLD1 genes and construction method of plasmid vector
A plasmid vector and construction method technology, applied in the field of biomedicine, can solve problems such as instability and achieve high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
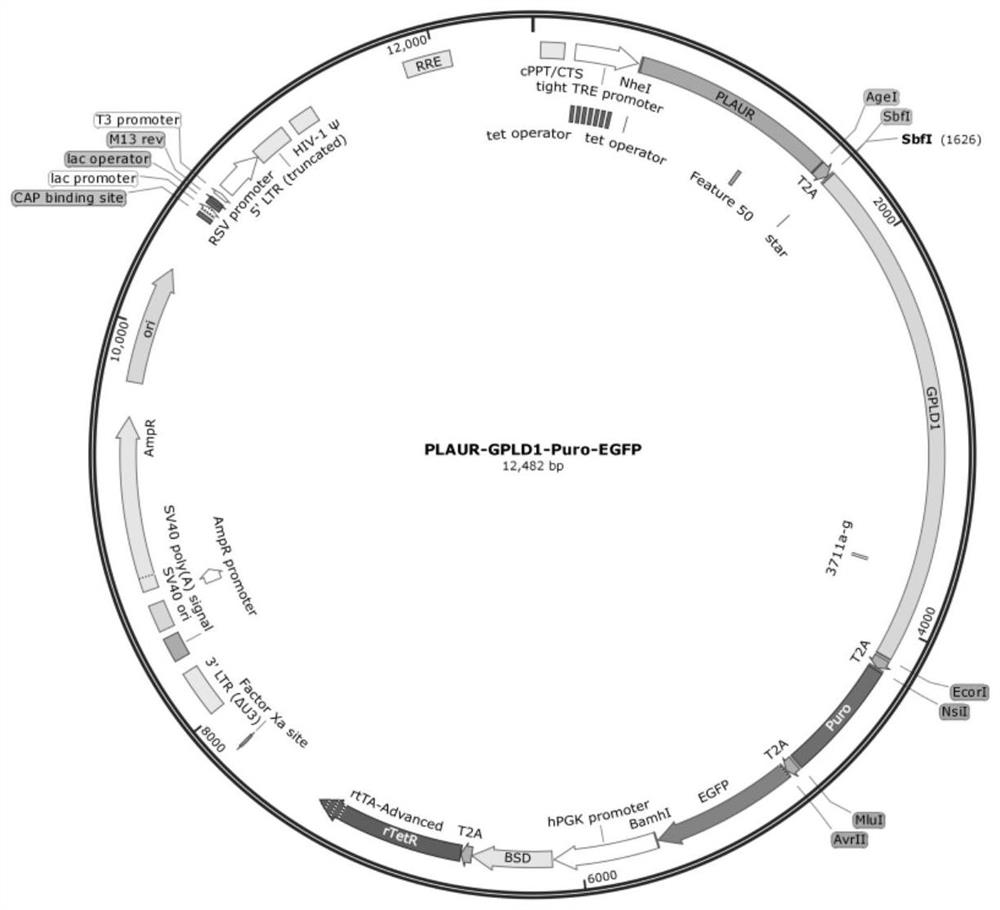
[0032] Example 1: Construction of PLAUR-T2A-GPLD1-T2A-Puro-T2A-EGFP plasmid
[0033] The commercial pCW-Cas9-Blast plasmid (Addgen, 83481); the original plasmid of green fluorescent protein (EGFP) was purchased from Addgene; the original plasmid of Puro resistance was purchased from Addgene.
[0034] All restriction enzymes and T4 ligase were purchased from Thermo Fisher Scientific; plasmid mini-extraction and purification kits, DNA gel recovery and purification kits were purchased from Axygen; chemically competent cells DH5α strain: purchased from Shanghai Sangong Bioengineering Ltd.
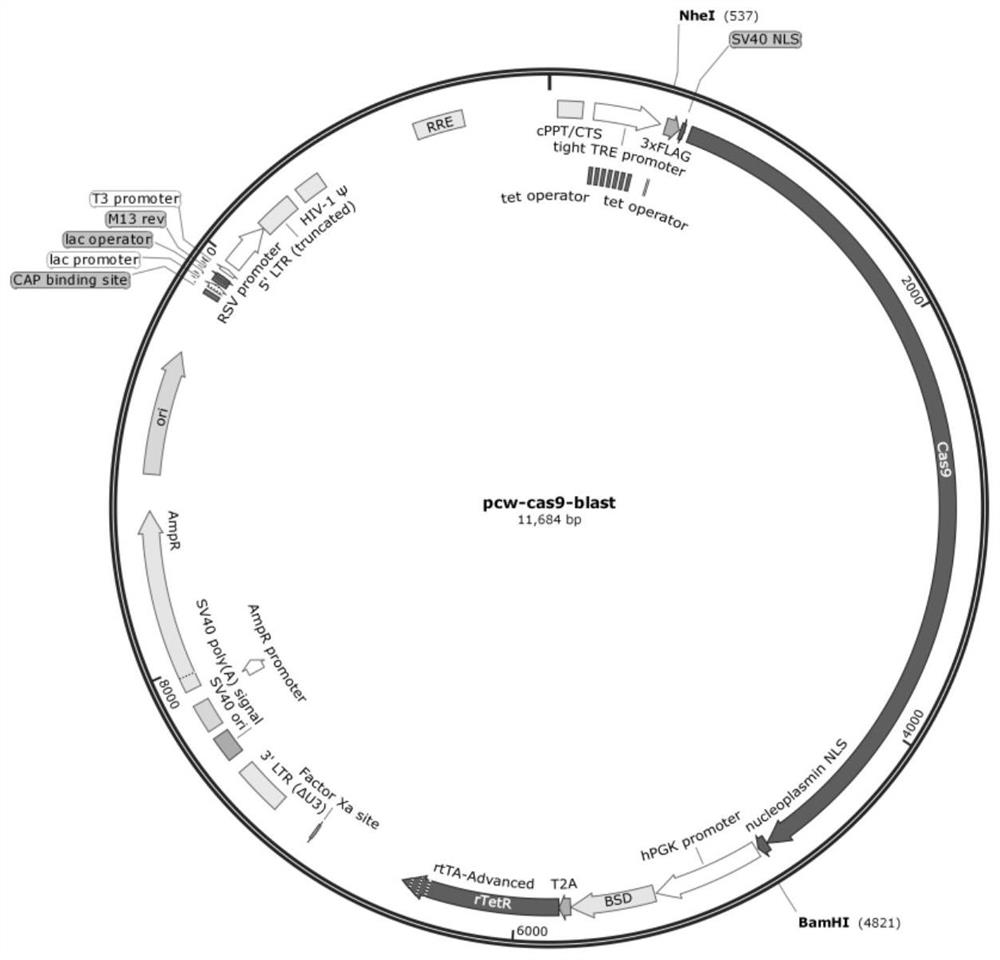
[0035] 1.1 Transformation of pCW-Cas9-Blast vector
[0036] The reason for choosing pCW-Cas9-Blast as the vector is that NheI, AgeI, SbfI, EcorI, NsiI, MluI, AvrII, and BamhI are single enzyme cutting sites in this vector, and it has a tetracycline-induced expression system.
[0037] Artificially synthesize a piece of DNA, the fragment contains the restriction endonuclease NheI-AgeI-SbfI-Ecor...
Embodiment 2
[0064] Example 2: Packaging of PLAUR-T2A-GPLD1-T2A-Puro-EGFP lentivirus
[0065] In the following examples, the culture medium used has:
[0066] The culture medium of HEK293T cells is composed of: 10% fetal bovine serum (purchased from HyClone, SH30396.03); 1% PS (purchased from Invitrogen, 10378016); 1% non-essential amino acid NEAA (purchased from Invitrogen, 11140050) ; The balance is DMEM (purchased from Invitrogen, 11965).
[0067] HEK293T cells were inoculated into six-well plates, cultured in DMEM medium containing 10% fetal bovine serum, and transfected when the confluence of the cells reached 70%-80%.
[0068] PLAUR-T2A-GPLD1-T2A-Puro-EGFP (20 μg), pVSVG (10 ug), and psPAX2 (15 ug) were co-transfected into HEK293T cells according to the instructions of Lipofectamine 2000.
[0069] After 6 h, the culture medium was replaced with DMEM containing 10% fetal bovine serum.
[0070] After continuing to cultivate for 60 hours, the culture solution was taken and centrifuge...
Embodiment 3
[0073] Example 3: Infection of HEK293T cells
[0074] Inoculate HEK293T cells into a six-well plate, culture with DMEM medium containing 10% fetal bovine serum, and add virus to infect when the cell confluence reaches 70%-80%. After 24 hours of infection, the culture medium was replaced with fresh DMEM culture medium containing 10% fetal bovine serum (containing tetracycline hydrochloride Dox+puromycin Puro at a final concentration of 2ug / ml) for screening. After 2-3 days of selection, a transformation efficiency of about 30% can be obtained. Then, the cell line expressing EGFP was obtained by sorting by flow cytometry, that is, the PLAUR-GPLD1 cell line was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com