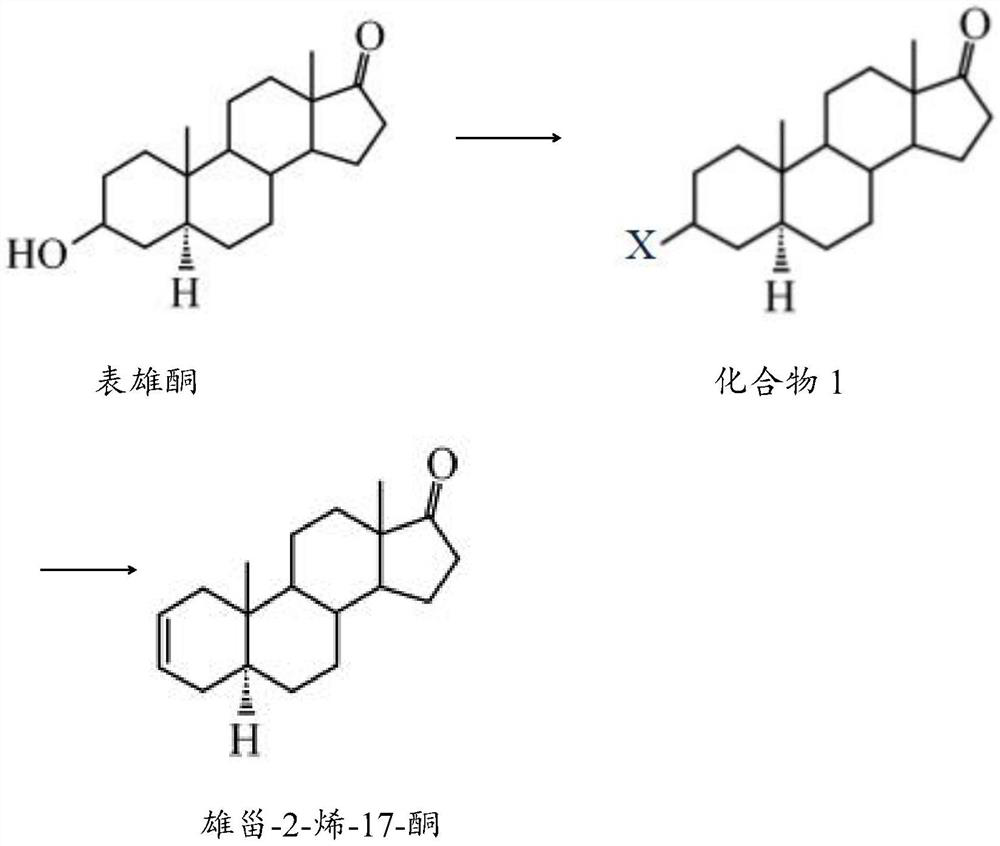
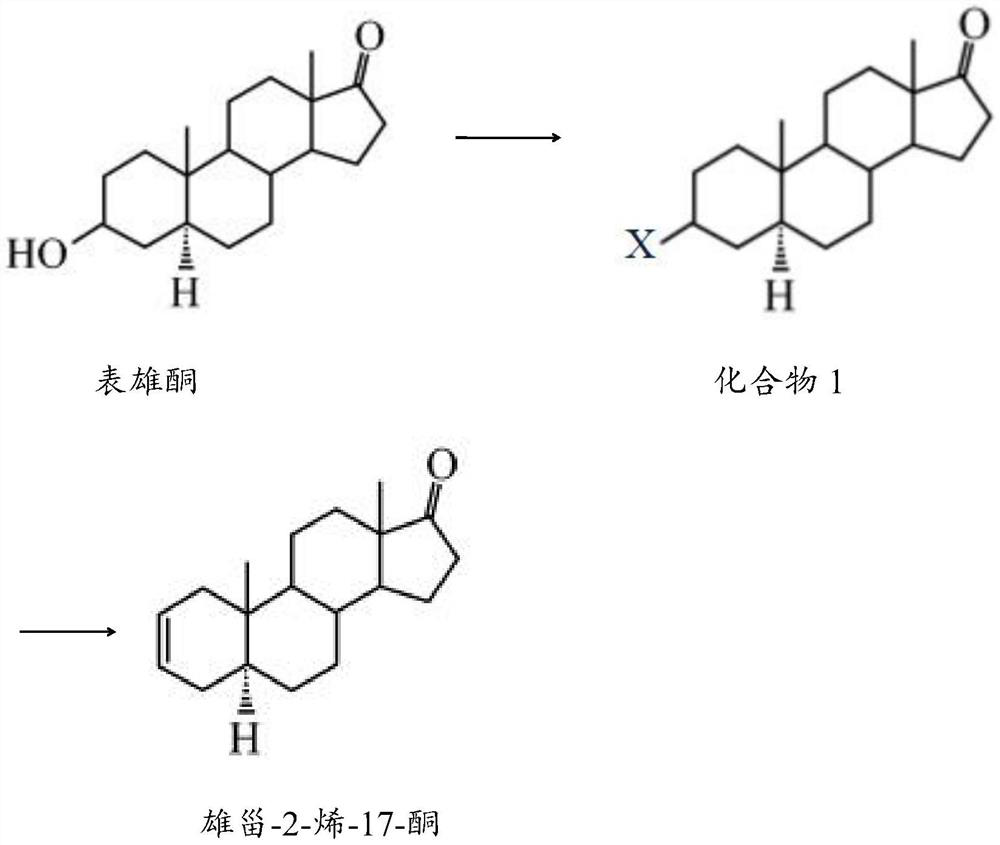
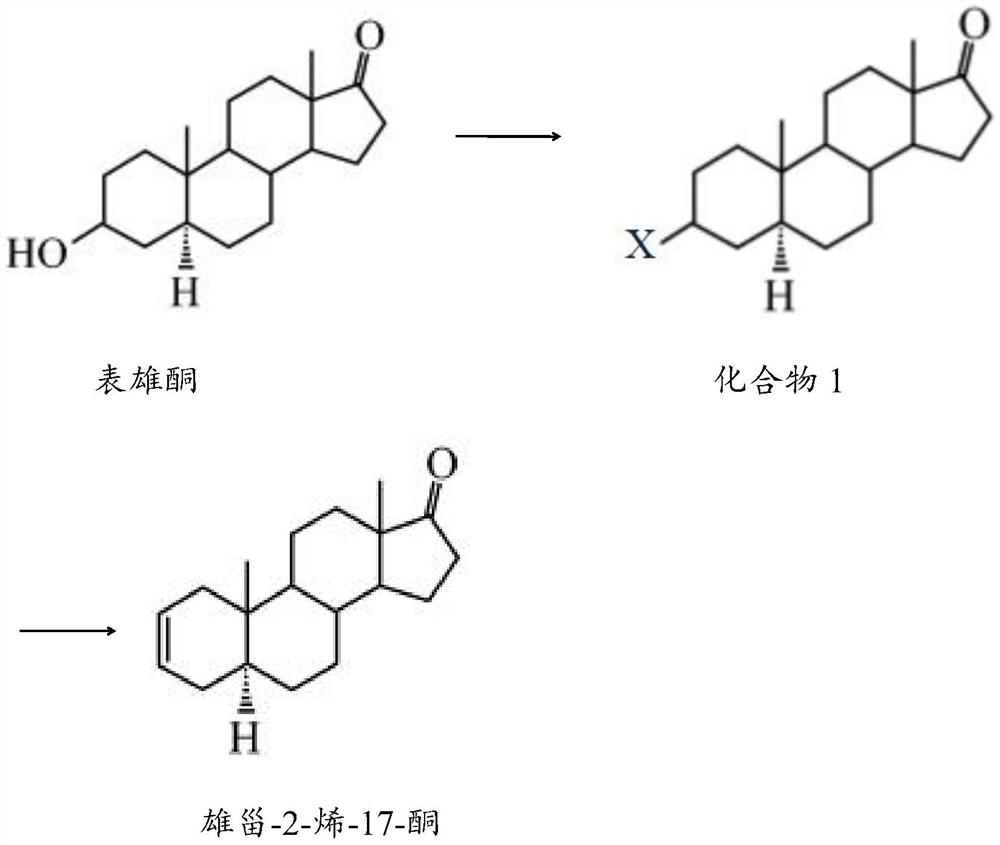
Preparation method of androst-2-ene-17-one
A technology for androsteroid and epiandrosterone, which is applied in the field of steroid drug intermediates, can solve the problems of harsh reaction conditions, low product yield, low purity and the like, and achieves the effects of low production cost, good reaction selectivity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of the androst-2-en-17-one comprises the following steps:
[0024] 1) After mixing epiandrosterone, a halogen-containing compound, a catalyst and a solvent, a halogenation reaction is carried out to obtain compound 1;
[0025] 2) Compound 1, a catalyst and a solvent are mixed for elimination reaction to obtain androst-2-en-17-one.
[0026] The halogen-containing compound in step 1) of the present invention preferably comprises bromosuccinimide, N-bromosuccinimide or carbon tetrachloride.
[0027] The catalyst in step 1) of the present invention is preferably triphenylphosphine; the solvent preferably includes one or more of dichloromethane, acetone, dimethylsulfoxide and N,N-dimethylacetamide; when When the solvent contains several components at the same time, each component is preferably mixed in an equal volume ratio.
[0028] The mass volume ratio of epiandrosterone, halogen-containing compound, catalyst and solvent in step 1) of the present ...
Embodiment 1
[0035] Add 5g of epiandrosterone, 9g of bromosuccinimide and 9g of triphenylphosphine into a reaction kettle filled with 155mL of dichloromethane, mix well, perform halogenation reaction at 45°C for 4h, and confirm the end point of the reaction by TLC. After the reaction was completed, the reaction solution was cooled to room temperature, washed with ethanol solution to remove triphenylphosphine, then washed twice with 100 mL saturated aqueous sodium bicarbonate solution, concentrated under reduced pressure, and dried with anhydrous sodium sulfide to obtain compound 1.
[0036] Mix 6.5g of compound 1, 3g of lithium carbonate and 6g of lithium bromide in 125mL of ethyl acetate, eliminate the reaction at 55°C for 7h, and confirm the end point of the reaction by TLC. After the reaction was completed, the reaction solution was cooled to room temperature, filtered, and washed with a 25% hydrochloric acid solution to remove lithium carbonate and lithium bromide, then washed twice wit...
Embodiment 2
[0039] Add 6.5g of epiandrosterone, 13g of carbon tetrachloride and 11g of triphenylphosphine into a reaction kettle filled with 90mL of acetone and 85mL of dimethyl sulfoxide mixed solvent, mix well, and conduct a halogenation reaction at 55°C for 2h. TLC confirmed the end of the reaction. After the reaction was completed, the reaction liquid was cooled to room temperature, washed with ethanol solution to remove triphenylphosphine, then washed twice with 130 mL saturated aqueous sodium bicarbonate solution, concentrated under reduced pressure, and dried with anhydrous sodium sulfide to obtain compound 1.
[0040] Mix 7.5g of compound 1, 3g of lithium carbonate and 9g of lithium bromide in 145mL of N,N-dimethylacetamide, eliminate the reaction at 65°C for 5h, and confirm the end of the reaction by TLC. After the reaction was completed, the reaction solution was cooled to room temperature, filtered, and washed with a 27% hydrochloric acid solution to remove lithium carbonate an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com