Kanamycin grafted cellulose-based antibacterial material and preparation method thereof
A cellulose-based, antibacterial material technology, applied in the direction of antibacterial drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low antibacterial activity and limit the application of chitosan/cellulose materials and other problems, to achieve the effect of simple reaction, reducing water pollution problems, and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Disperse 5 g of α-cellulose and 0.058 g of kanamycin sulfate in 10 mL of water, add 5 μL of 0.1 M hydrochloric acid dropwise, and stir for 30 minutes; under dark conditions, add 0.05 g of glutaraldehyde dropwise, and stir at room temperature for 2 h; filter, Collect the particles, remove incompletely reacted kanamycin sulfate with a large amount of pure water, and dry in vacuum at 60° C. for 5 hours to obtain a cellulose-based antibacterial material grafted with kanamycin sulfate.
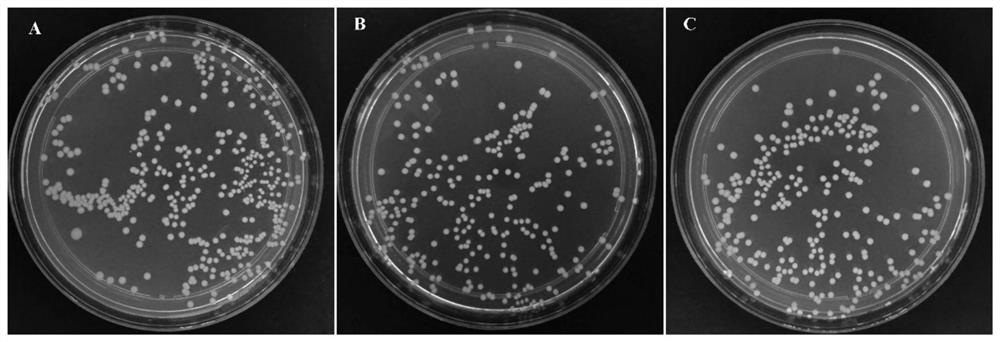
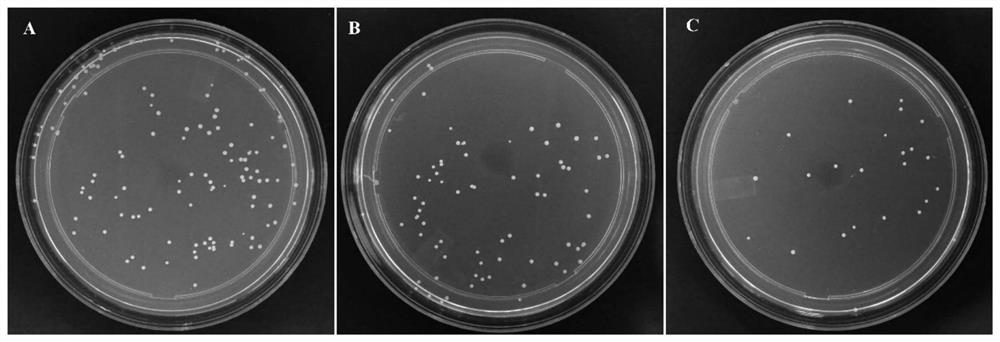
[0039] Detect the inhibitory effect of the cellulose-based antibacterial material of this embodiment on Escherichia coli (Gram-negative bacteria): Inoculate Escherichia coli into a liquid medium (LB medium), cultivate at 37°C, until the bacterial liquid OD 600 =1.39, which is the original bacterial liquid. Take 1mL of the original bacterial liquid, add 50mg of the cellulose-based antibacterial material of this example or α-cellulose, shake at 37°C for 3h, smear the plate, and detect the numb...
Embodiment 2
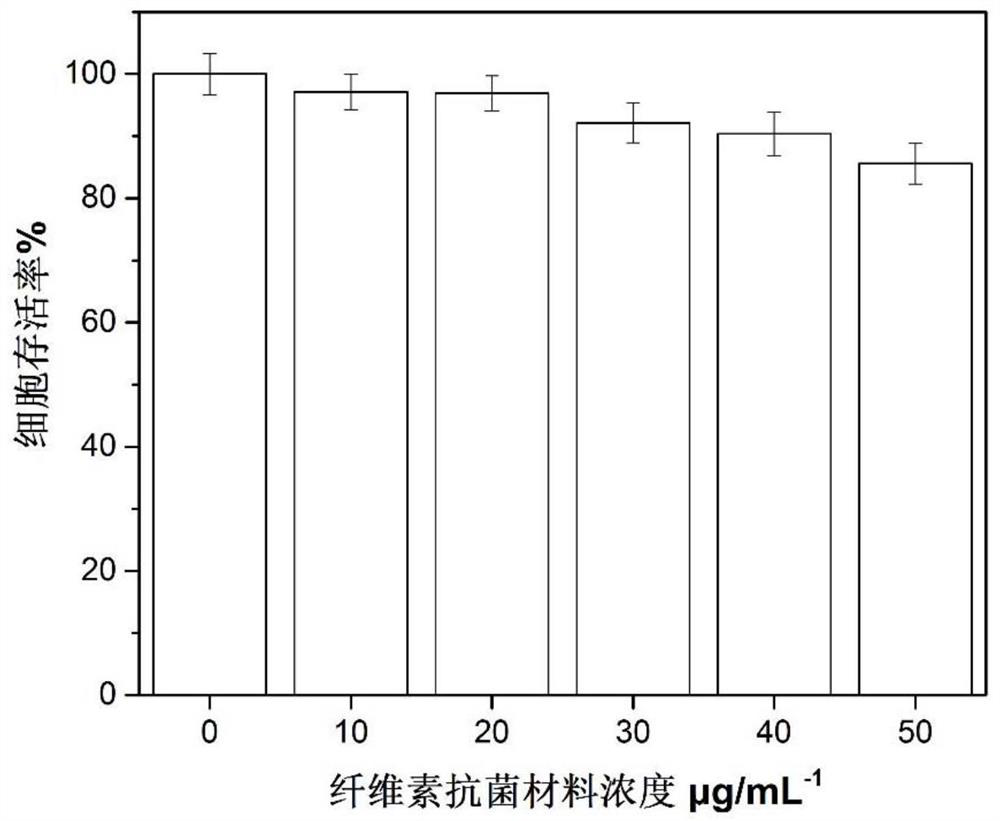
[0041] Disperse 5g of cotton cellulose and 5.8g of kanamycin hydrochloride in 50mL of water, add dropwise 2.5mL of 0.1M acetic acid aqueous solution, and stir for 30 minutes; under dark conditions, add dropwise 5g of adipaldehyde, and stir at room temperature for 6h; Filter, collect particles, remove incompletely reacted kanamycin hydrochloride with a large amount of pure water, and vacuum dry at 40°C for 10 hours to obtain a cellulose-based antibacterial material grafted with kanamycin hydrochloride, with a BSA adsorption capacity of 162.5 mg / g.
[0042]Detect the inhibitory effect of the cellulose-based antibacterial material of this embodiment on Staphylococcus aureus (Gram-positive bacteria): inoculate Staphylococcus aureus into a liquid medium (LB medium), cultivate at 37 ° C, until the bacterial liquid OD 600 =1.18, which is the original bacterial liquid. Take 1mL of the original bacterial liquid, add 50mg of the cellulose-based antibacterial material of this example or ...
Embodiment 3
[0046] Disperse 5g of pulp (without water, cellulose content > 85%) and 2g of kanamycin sulfate in 20mL of water, add dropwise 500μL of 0.5M phosphoric acid aqueous solution, and stir for 1 hour; Dialdehyde, stirring at room temperature for 12 hours; filtering, collecting particles, removing incompletely reacted kanamycin sulfate with a large amount of pure water, and vacuum drying at room temperature for 24 hours to obtain a cellulose-based antibacterial material grafted with kanamycin sulfate.
[0047] Detect the inhibitory effect of the cellulose-based antibacterial material of this embodiment on Salmonella (Gram-negative bacteria): inoculate Salmonella into a liquid medium (LB medium), cultivate at 37 ° C, until the solution OD 600 = 2.19. Take 1mL of the original bacterial liquid, add 50mg of the cellulose-based antibacterial material or paper pulp of this example, shake at 37°C for 3h, smear the plate, and detect the number of Salmonella growing on the nutrient agar plat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com