Radioactive < 18 > F labeled compound and application thereof
A radioactive and chemical compound technology, applied in the field of medical imaging probes, can solve the problems of low penetration depth of fluorescent probes, easy quenching of optical signals, and difficult clinical application, etc., and achieves commercial application and clinical promotion, high sensitivity , good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis and Labeling of Compound 1
[0038] The structural formula of compound 1 is
[0039] The synthetic route of compound 1 is as follows:
[0040]
[0041] Specifically include the following steps:
[0042] (1) Accurately weigh 13.8g of 4-chloronitrobenzene, 25g of diethanolamine and 41.4g of potassium carbonate into a 250mL round bottom flask, and reflux at 120°C for 4h. After the reaction, it was cooled to room temperature, and slowly added dropwise into 500 mL of deionized water. The resulting aqueous solution was filtered and extracted three times with 1.5 L of ethyl acetate, and the solvent was removed from the extracted ethyl acetate solution by rotary evaporation to obtain a red crude product. The crude product was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain compound 5.
[0043] (2) Accurately weigh 2.26g of compound 5 and dissolve in 20mL of dichloromethane, and add 2.01g of triethylamine. The mixed...
Embodiment 2
[0047] Example 2: Synthesis and Labeling of Compound 2
[0048] The structural formula of compound 2 is
[0049] The synthetic route of compound 2 is as follows:
[0050]
[0051] Specifically include the following steps:
[0052] (1) Accurately weigh 10g of 3,4-dinitrochlorobenzene, 3.48g of potassium carbonate and 10g of diethanolamine in a 100mL round bottom flask, and react at 50°C for 10min. After the reaction, the reaction solution was diluted with 100 mL of methanol and the crude product was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain compound 8.
[0053] (2) Accurately weigh 3.14 g of compound 8, dissolve it in 20 mL of dichloromethane, add 3.03 g of triethylamine, and stir at room temperature for 30 min. The reaction solution was added dropwise to a solution of thionyl chloride in dichloromethane (2.61 g in 5 mL of dichloromethane) in an ice-water bath. After the dropwise addition was completed, it was heated to reflux for...
Embodiment 3
[0056] Example 3: Nitroreductase Reduction of Compound 1
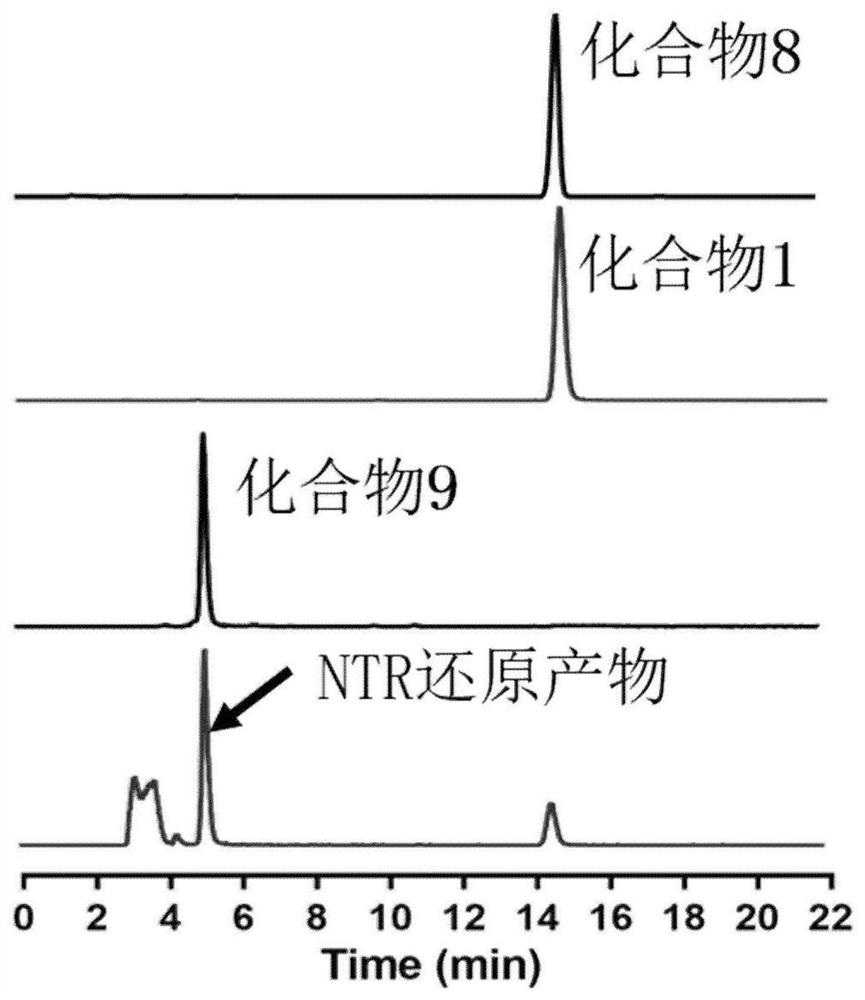
[0057] 10 μg / mL nitroreductase solution (100 μL), 200 μM NADPH (100 μL) and 500 μCi compound 1 injection (20 mL) were added to 0.5 mL pH 7.4 Tris buffer solution. The mixed solution was incubated at 37° C. for 3 h under nitrogen protection. The reacted solution (100 μL) was loaded into HPLC for analysis. HPLC conditions: A phase: deionized water; B phase: acetonitrile; A: B = 50%: 50%; 1mL / min.
[0058] From the results of HPLC analysis, it can be seen that after compound 1 was incubated with nitroreductase for 3 hours, 88% of the original drug was converted into other substances, and 37% of it was reduced, that is, the nitro group on the molecule was reduced to an amino group, which was absorbed by the nitroreductase. Electron groups become electron donating groups. Illustrate that the probe compounds in the examples of the present invention can be reduced under the action of nitroreductase, and the specific result...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com