Two-phase oil adjuvant foot-and-mouth disease vaccine stabilizer and application thereof
A foot-and-mouth disease vaccine and stabilizer technology, applied in the field of animal biological products, can solve the problems of reduced immune effect, undetectable antibodies, and demulsification, so as to reduce animal stress, ensure immunogenicity, and increase the proportion of water phase Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
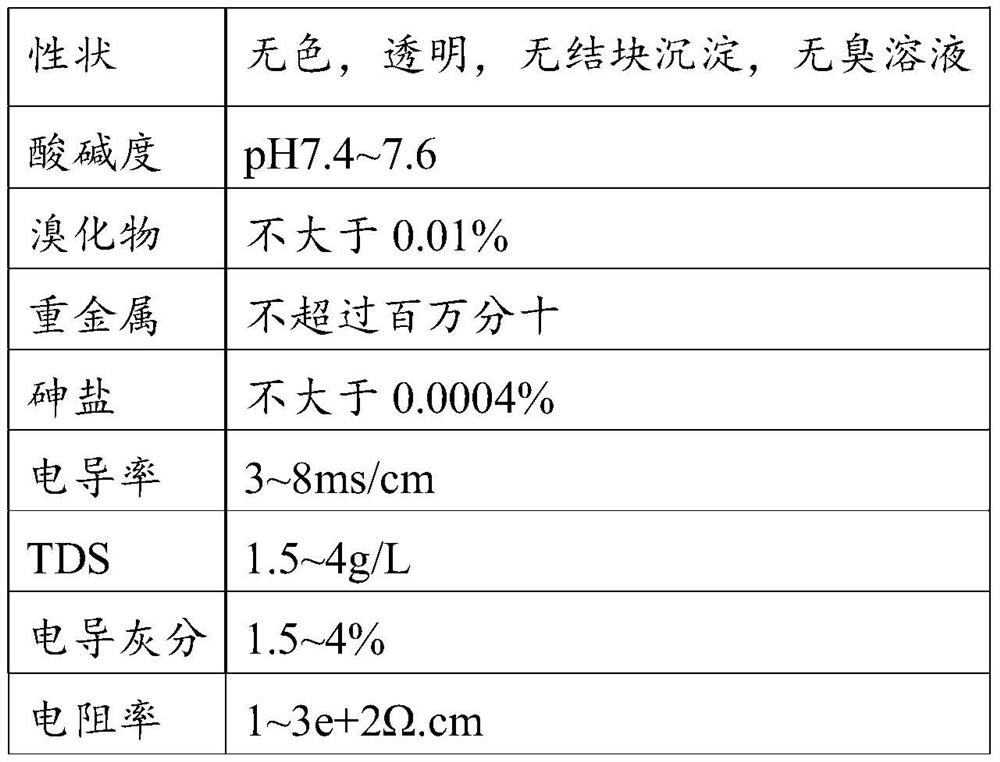
[0025] As a preferred embodiment of the stabilizer of the present invention, the acid comprises tartaric acid, citric acid, lactic acid, malic acid, maleic acid, sorbic acid, succinic acid, ricin alcohol, citric acid; the alcohol includes Alcohol, elevale alcohol, dodecyl alcohol (lauryl), tetrahydroganol (nargol), hexadecanol (palmitol), eaterseanol (stearol), duethanol (peanut alcohol) ), Twenty di alcohol (hull alcohol); the amine such as glutamine, asparagine, nicotinamide; the charge modifier comprises sodium chloride, potassium chloride, ammonium chloride, monoethanolamine chloride, Diethanolamine chloride, disodium hydrogen phosphate, sodium phosphate, sodium triphosphate, sodium stearate, magnesium stearate, gluconate, acetate, citrate; the polymer includes hydroxyethyl Cellulose, polyethylene glycol (PEG), glucan, acrylate, and copolymers thereof, polyveridate, polyvinyl alcohol, polyacrylic acid, and sodium salt, chitosan, natural glue, and modifiers such as Algic acid a...
Embodiment 1
[0033] Example 1, a stabilizer for a foot-and-mouth disease vaccine applied to a double photograph oil?
[0034] The stabilizer of the footheroid vaccine applied to the double-phase oil rejuperature of this embodiment consists of a substance of the following mass percentage:
[0035] Acid: 0.2% 酒 石 石, 0.1% of sorghum acid,
[0036] Alcohol: 0.3% decanol, dodecylol (lauryl) 0.2%,
[0037] Amine: 0.3% of glutamine,
[0038] Polymer: 1% of sodium carboxymethylcellulose, potassium alginate 0.5%,
[0039] Charge regulator: 0.5% of potassium chloride, 0.5% sodium octanoate,
[0040] Sugar: 2% lactose, 2% mannose, 5% of dimethyl-β-cyclodextrin,
[0041] Water for injection: 87.15%.
Embodiment 2
[0042] Example 2, a stabilizer of a foot-and-mouth hyperplate applied to a double photographic oil joiner
[0043] The stabilizer of the footheroid vaccine applied to the double-phase oil rejuperature of this embodiment consists of a substance of the following mass percentage:
[0044] Acid: 0.2% linoleic acid, 0.1% succinate,
[0045] Alcohol: Eateginol (stearol) 0.2%,
[0046] Amine: nicotinamide 0.3%, 5.1% of glutamine,
[0047] Polymer: 1%, polyethylene glycol (40) hydrogenated castor oil 1.5%,
[0048] Charge regulator: 0.5% of hydrogen phosphate, 0.04% podium phosphate, 0.1% of potassium chloride, 0.5% gluconate,
[0049] Sugar: 2% maltose, 2% fibrous diose, 3% of hydroxypropyl-β-cyclodextrin,
[0050] Water of injection: 88.46%,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com