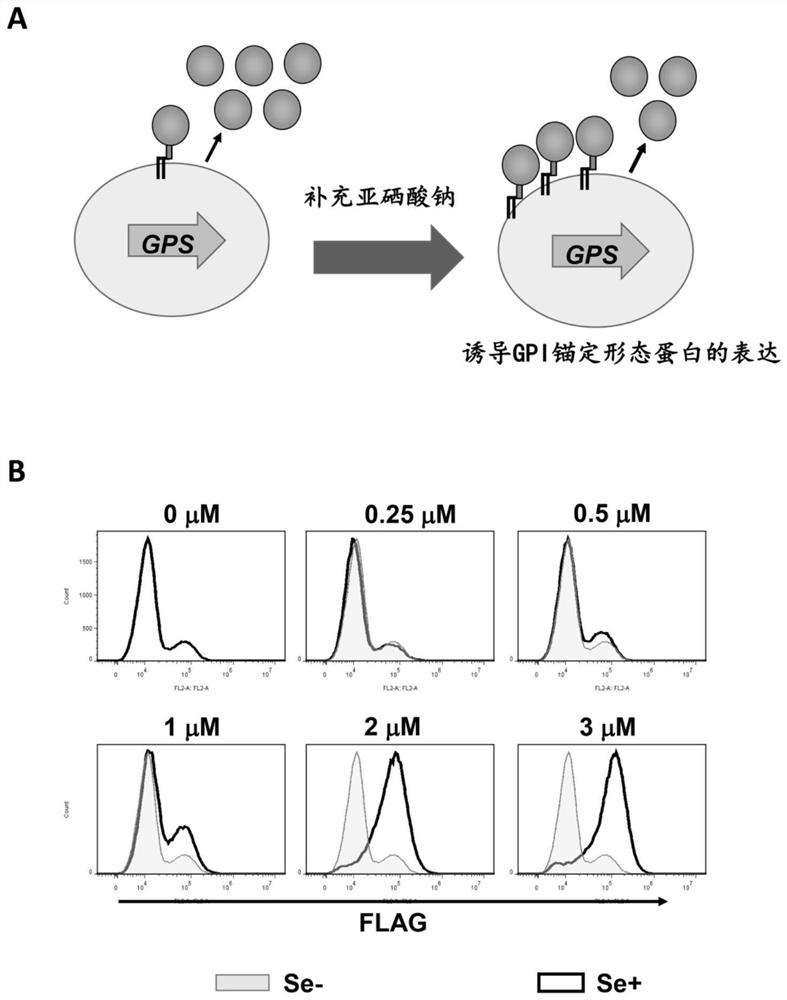
GPI anchoring protein expression system containing selenocysteine and cell with high-expression of recombinant protein
A selenocysteine and anchoring protein technology, applied in the direction of genetically modified cells, peptides containing affinity tags, peptides containing His tags, etc., can solve problems such as darkening of nails and skin, hair loss, etc., to achieve Simplify the difficulty and filter the effect of convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
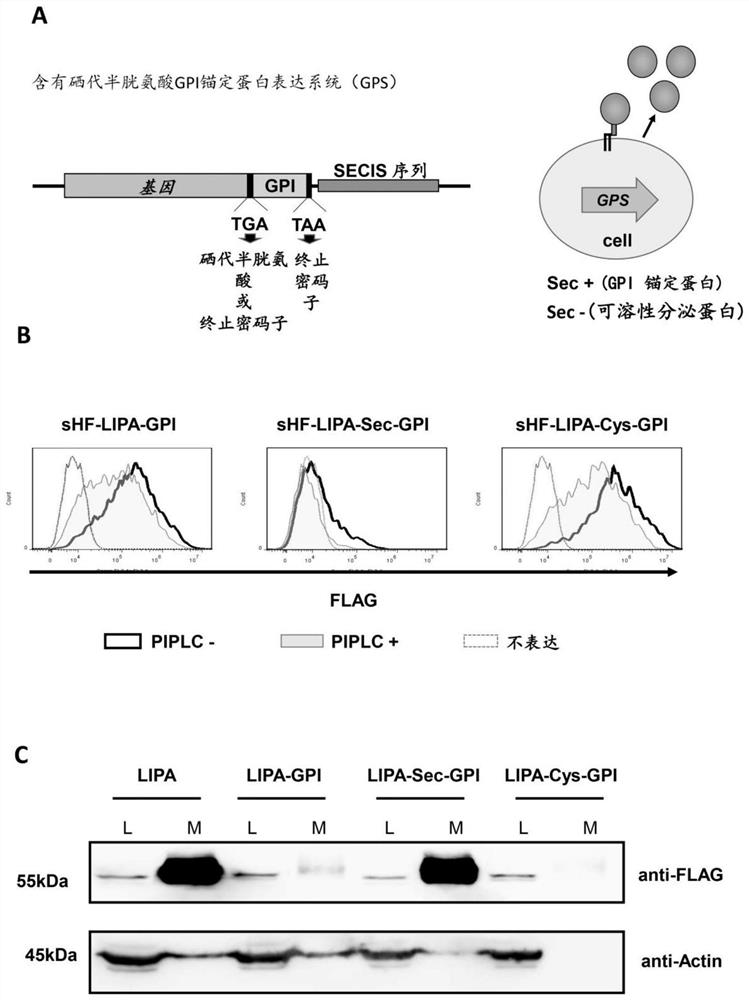
[0032] Construction of a GPI-anchored protein expression system containing selenocysteine (taking recombinant lysosomal acid lipase (LIPA) as an example) by combining the oxidoreductase protein family selenocysteine and GPI Ankyrin synthesis features are constructed.
[0033] Prepare the vector: prepare the vector pME-Puro-sHF-GPI, which has a puromycin resistance gene (Puro), a his and Flag tag gene (sHF) with a signal peptide, and a GPI modified signal sequence;
[0034] The pME-Puro-sHF-GPI was connected to LIPA (lysosomal acid lipase) by In-fusion homologous recombination to generate the plasmid pME-Puro-ssHF-LIPA-GPI, and the stop codon UGA (in the environment containing selenium The codon for the expressive selenocysteine Sec below) was inserted before the GPI attachment signal sequence of pME-Puro-sHF-LIPA-GPI (sHF represents the endoplasmic reticulum signal sequence plus the His6-FLAG-tag ) by site-directed mutagenesis of CTAATGAGGAAATATCAGTGACTCGAGAATGGTGGGACA ...
Embodiment 2
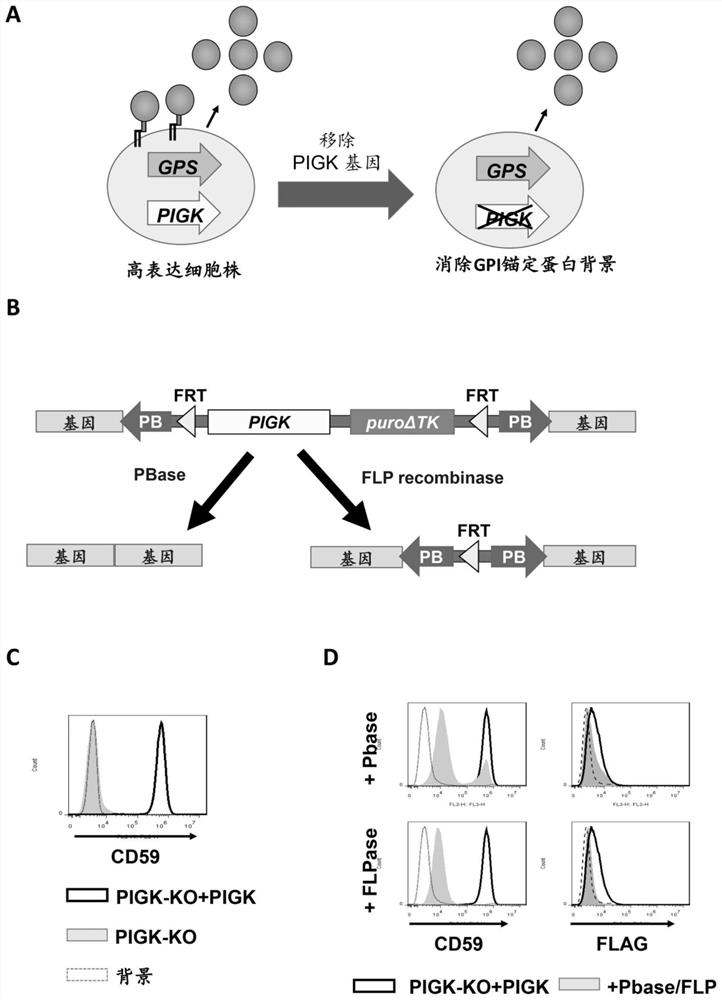
[0042] Construction of a system for removing the PIGK gene: First, the PIGK (a catalytic subunit of the GPI-anchored protein transamidase complex) gene was knocked into HEK293 cells using the CRISPR / Cas9 system. BbsI cut the vector pX330-EGFP (purchased from Addgen, purchase link https: / / www.addgene.org / 42230 / ). The PIGK-KO target sequence group caccGCTCTTGTCCTTCGGCAGCGTGG and aaacCCACGCTGCCGAAGGACAAGAGC were ligated into the digested pX330-EGFP to generate pX330-EGFP-PIGK-KO, and the protein produced by the plasmid knocked out of the PIGK gene could not be anchored by GPI after transfection into cells modified, all secreted into the culture medium and become soluble proteins. After transfection, cells with EGFP were sorted with a cell sorter S3 (Bio-Rad Laboratories). The collected cells were cultured for 7 days or more and subjected to limiting dilution to obtain clonal KO cells. The clone without WT allele was selected, and the DNA sequence was analyzed by sequencing. The...
Embodiment 3
[0050] Validation of elimination of GPI-form recombinant proteins by knocking out the PIGK gene: Even though we obtained cell lines highly expressing recombinant proteins using the GPS system, some recombinant proteins were expressed on the cell surface in the form of GPI-AP. When administered as a drug, the small fraction of GPI-anchored forms may affect protein properties such as activity and immunogenicity. We therefore next attempted to eliminate the GPI-anchored form from cells.
[0051] For this purpose, we established cell lines that can regulate the expression of the GPI biosynthesis gene PIGK. Deletion of endogenous PIGK from HEK293 cells resulted in loss of GPI-AP expression (attached image 3 A). Then, a cassette containing the PIGK gene fragment and the puroΔTK gene flanked by FRT sites and PB transposon recognition sites was reinserted into the genome to restore GPI-AP expression in the rescued cells. In this system, PIGK can be excluded by FLP recombinase or P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com