Preparation method for synthesizing diaryl thioether compound based on L-cysteine
A technology of diaryl sulfide and cysteine, which is applied in the intermediate field of medicine and chemical industry, can solve the problems of inflammability and explosion, and achieve the effects of good compatibility, mild reaction conditions, great use value and social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 4,4'-dimethyl diphenyl sulfide (1)
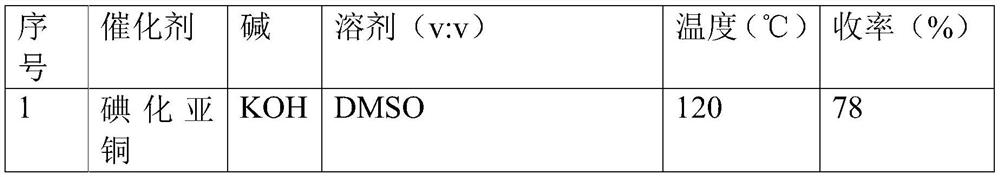
[0026] Put 109.0 mg (0.5 mmol) of 4-iodotoluene, 30.3 mg (0.25 mmol) of L-cysteine, 0.5 mmol of copper salt, 1.5 mmol of alkali, and 1.5 mL of solvent into a 15 mL reaction tube equipped with a magnet, Seal it with argon, heat and stir, and react for 12 hours. After the reaction, after the reaction solution was cooled to room temperature, the reaction solution was washed with 15 ml of water and transferred to a 250 ml separatory funnel, extracted three times with ethyl acetate, the organic layers were combined, the water layer was removed, and the ethyl acetate layer was washed with saturated salt After washing with water three times, it was dried by adding anhydrous magnesium sulfate. After rotary evaporation under reduced pressure and column chromatography (the mobile phase condition is petroleum ether), white crystals were obtained, which were the corresponding products shown in Formula 1. 1 H NMR (600M...
Embodiment 2
[0033] Embodiment 2: Preparation of 4,4'-diaminodiphenylsulfide (2)
[0034] 4-iodoaniline 109.5mg (0.5mmol) and L-cysteine 30.3mg (0.25mmol), cuprous iodide 95.2mg (0.5mmol), potassium hydroxide 84.2mg (1.5mmol), 1.5mL solvent DMSO / DMF=4:1 (v:v), placed in a 15mL reaction tube equipped with a magnet, sealed with argon, heated and stirred, and reacted in an oil bath at 120°C for 12 hours. After the reaction, after the reaction solution was cooled to room temperature, the reaction solution was washed with 15 ml of water and transferred to a 250 ml separatory funnel, extracted three times with dichloromethane, the organic layers were combined, the water layer was removed, and the ethyl acetate layer was washed with saturated salt After washing with water three times, it was dried by adding anhydrous magnesium sulfate. Rotary evaporation under reduced pressure and column chromatography (mobile phase conditions: petroleum ether / ethyl acetate = 10:1 (v / v)) obtained 30.9 mg of a...
Embodiment 3
[0037] Embodiment 3: Preparation of 3,3'-dimethyl diphenyl sulfide (3)
[0038] 3-iodotoluene 109.0mg (0.5mmol) and L-cysteine 30.3mg (0.25mmol), cuprous iodide 95.2mg (0.5mmol), potassium hydroxide 84.2mg (1.5mmol), 1.5mL solvent DMSO / DMF=4:1 (v:v), placed in a 15mL reaction tube equipped with a magnet, sealed with argon, heated and stirred, and reacted in an oil bath at 120°C for 12 hours. After the reaction, after the reaction solution was cooled to room temperature, the reaction solution was washed with 15 ml of water and transferred to a 250 ml separatory funnel, extracted three times with ethyl acetate, the organic layers were combined, the water layer was removed, and the ethyl acetate layer was washed with saturated salt After washing with water three times, it was dried by adding anhydrous magnesium sulfate. After rotary evaporation under reduced pressure and column chromatography (the mobile phase condition is petroleum ether), 41.4 mg of a yellow solid represent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com