Thermally activated delayed fluorescent polymer and application thereof
A fluorescent polymer, thermal activation delay technology, used in luminescent materials, drug combinations, photodynamic therapy, etc., can solve the problems of fluorescence quenching, loss of photodynamic efficiency, leakage, etc., to achieve easy operation, improve diagnosis and treatment efficiency, The effect of high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
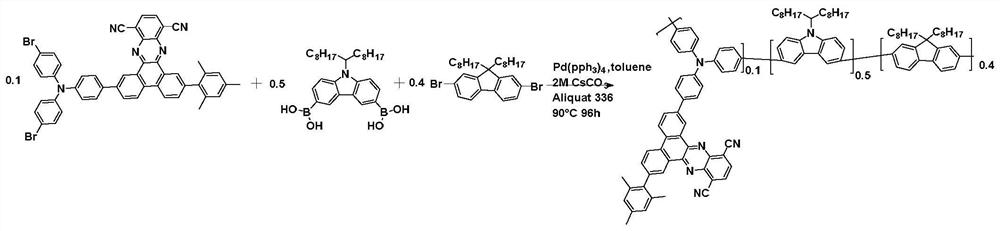
[0071] The preparation method of the integrated nano-reagent for diagnosis and treatment based on a heat-activated delayed fluorescent polymer includes: reprecipitation method, molecular micelle carrier, thin film dispersion method, reflux precipitation method and other common organic semiconductor nano-reagent preparation methods.
[0072] The method of reprecipitation method to prepare a kind of nano-reagent for activating delayed fluorescent polymer is as follows: first, organic semiconductor and functional coating agent (such as PSMA or DSPE-PEG2000, etc.) are dissolved in tetrahydrofuran (THF) respectively, and the concentration is 1 mg / mL solution. After stirring overnight under inert gas protection conditions, the coating agent solution was filtered with a 7 μm glass fiber filter to remove insoluble substances in the bath solution. Then, the above two solutions are mixed in a certain proportion.
[0073]For example, 1 mg / mL organic semiconductor and 0.2 mg / mL PSMA in ...
Embodiment 1
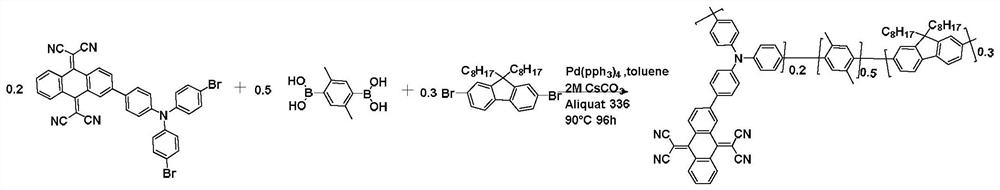
[0081] This embodiment provides a conjugated polymer A based on thermally activated delayed fluorescence. The structural formula of the conjugated polymer A is as follows:
[0082]
[0083] Formula A
[0084] The synthetic route of the conjugated polymer is shown in general formula A.
[0085] Take 0.40g (0.57mmol) 2,2'-(2-(4-(bis(4-bromophenyl)amino)phenyl)anthracene-9,10-dialkylene)dimethylnitrile, 0.28g (1.42mmol) (2,5-dimethyl-1,4-phenylene) diboronic acid and 0.47g (0.85 mmol) 2,7-dibromo-9,9-dioctyl-9H-fluorene were added to 50mL reaction tube, then add 2MCs to the reaction tube in sequence 2 CO 3 (aq) (1mL), 9.2mg (0.008mmol) catalyst tetrakistriphenylphosphine palladium, 5.3mg (0.013mmol) ligand methyl trioctyl ammonium chloride, 5mL anhydrous toluene, at 95 ℃ and argon protection Under the conditions, the reaction was stirred for 96 hours to obtain a polymer. After the polymer is cooled to room temperature, slowly pour it into 150mL of methanol to form a preci...
Embodiment 2
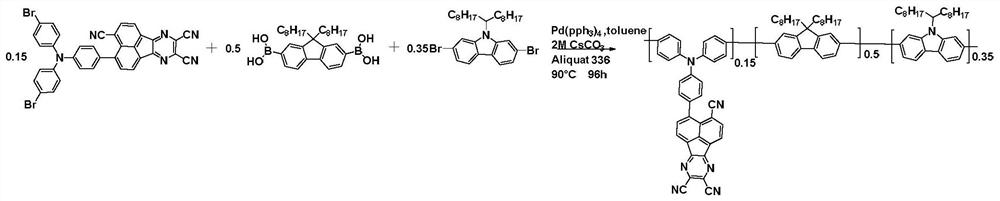
[0087] This embodiment provides a conjugated polymer B based on polycyclic aromatic hydrocarbons. The general structural formula B of the conjugated polymer is as follows:
[0088]
[0089] Formula B
[0090] The synthetic route of the conjugated polymer is shown in general formula B.
[0091] Take 0.50g (0.73mmol) 2,2'-(2-(4-(bis(4-bromophenyl)amino)phenyl)anthracene-9,10-dialkylene)dimethylnitrile, 1.17g (2.44mmol) (9,9-dioctyl-9H-fluorene-2,7-diyl)diboronic acid and 0.96 g (1.71mmol) 2,7-dibromo9-(heptadecan-9-yl) -9H-carbazole was added to a 50mL reaction tube, and 2MCs were added to the reaction tube in turn 2 CO 3 (aq) (1mL), 9.2mg (0.008mmol) catalyst tetrakis triphenylphosphine palladium, 5.3mg (0.013mmol) ligand methyl trioctyl ammonium chloride, 5mL anhydrous toluene, at 95 ℃ and argon protection Under the conditions, the reaction was stirred for 96 hours to obtain a polymer. After the polymer is cooled to room temperature, slowly pour it into 150mL of methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com