Recombinant III-type humanized collagen, nucleic acid, carrier and implant
A human collagen and collagen technology, applied in the field of genetic engineering, can solve the problems of easy degradation, poor stability of recombinant humanized collagen, limited application, etc., and achieve the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Structural Design of Recombinant Type III Humanized Collagen
[0045] According to the present invention, four peptide chains of natural human type III collagen α1 chain sequence (reference sequence Genebank accession number: NM_000090.3) 549-560aa, 597-613aa, 881-902aa, 648-662aa are spliced in order to form a monomer, See SEQ.ID.NO.5 for the amino acid sequence of the monomer.
[0046] In the present invention, the monomers composed of the above-mentioned tetrapeptide segments are used for repetitive tandem expression.
[0047] (2) Preparation of recombinant type III humanized collagen
[0048] 2.1 Acquisition of genes
[0049] The amino acid sequence of the above monomer is designed according to the codon preference of Pichia pastoris, and the corresponding nucleotide sequence is designed. At the same time, for subsequent molecular operations, the DNA sequence is added with Xho I restriction endonuclease sites CTCGAG and KEX2 at the 5' end Enzyme cutting sit...
Embodiment 2
[0057] Preparation of Monomeric Ten-repeat Recombinant Type III Humanized Collagen
[0058] (1) Construction of genetically engineered bacteria
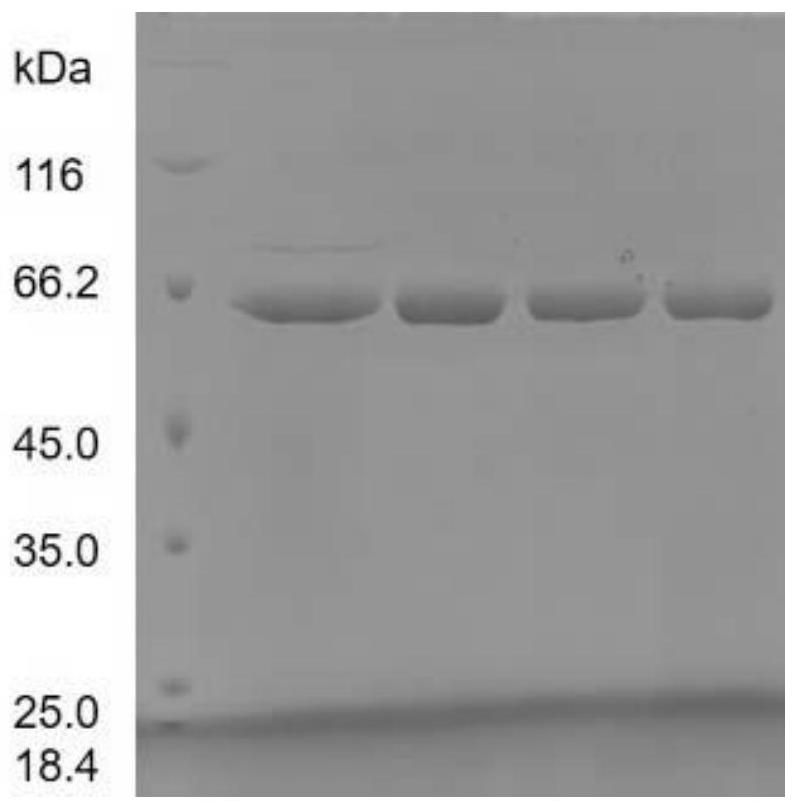
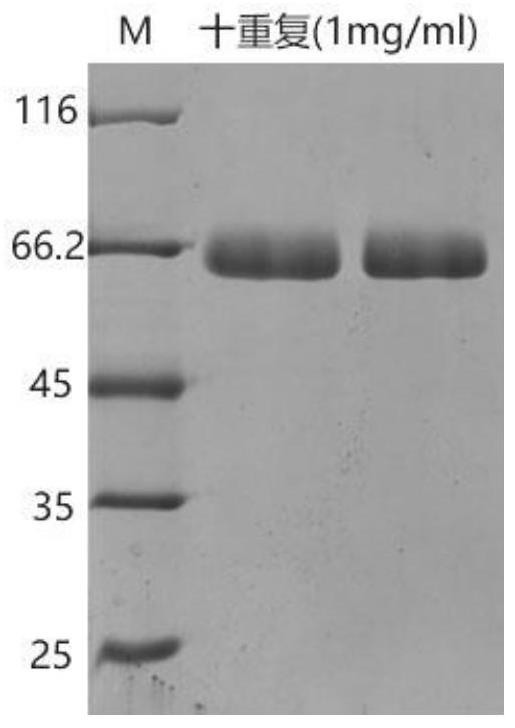
[0059] After extracting the multi-repeat tandem expression plasmid obtained in Example 1, preferably the ten-repeat tandem pPIC9K-COL3-10 plasmid, linearize it with restriction endonuclease Sal I, and then transform Pichia pastoris by electroporation Host strain GS115 (Invitrogen), high-copy transformants were screened by G418 (4mg / mL, YPD medium), and the expression was confirmed by shake flask fermentation (29°C, 200rpm, BMMY medium, methanol feed 1% at intervals of 24 hours) Happening. The shake flask fermentation supernatant was detected by SDS-PAGE electrophoresis, from figure 1 It can be seen from the figure that there is an obvious electrophoresis band at about 60KD, and it is confirmed that the recombinant type III humanized collagen genetically engineered bacteria with monomer ten repeats was obtained.
[0060] (2) Fermen...
Embodiment 3
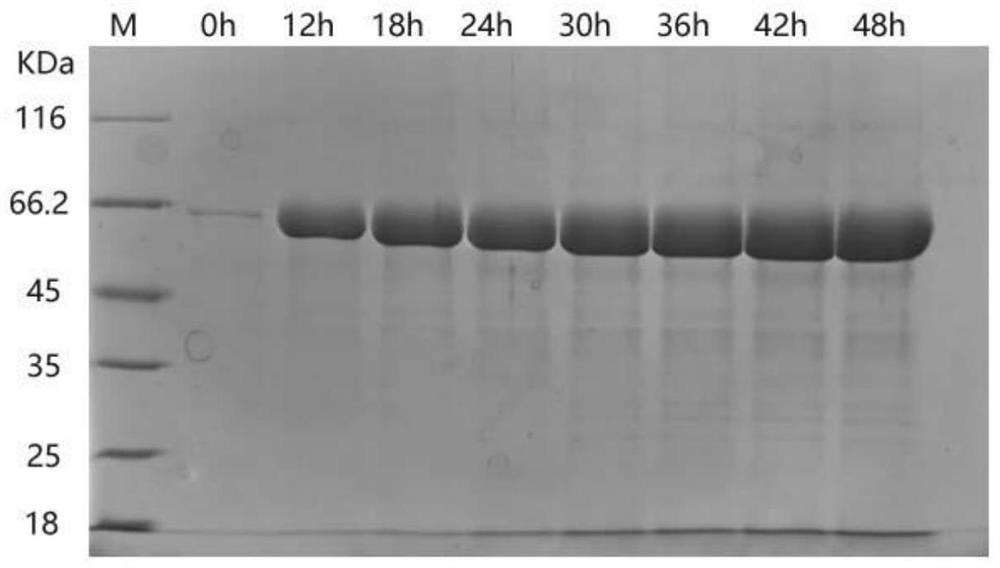
[0063] The obtained recombinant type III humanized collagen freeze-dried powder is prepared into a 0.1% liquid with purified water, sealed and subpackaged after sterile filtration, and placed under the conditions of a temperature of 40°C ± 2°C and a relative humidity of 75% ± 5%. Accelerated experiments were carried out at room temperature at 25°C±2°C, relative humidity 60%±5%, samples were taken at different time points, and the integrity of the recombinant type III humanized collagen was detected by SDS-PAGE electrophoresis. Test results such as Figure 4 shown; from Figure 4 It can be seen from the figure that at room temperature of 25°C, when samples were taken at 3 and 6 months, the protein bands were complete and there was almost no degradation; at accelerated conditions at 40°C, when samples were taken at 3 and 6 months, at Complete, almost no degradation, sampling in June, a little protein degradation. From the above experimental results, it can be concluded that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com