Injectable dextran hydrogel microsphere filler and preparation method thereof
A technology of hydrogel microspheres and dextran, which is applied in the field of medical cosmetology, can solve the problems of amine crosslinking agent residue, interference with emulsification stability, uneven emulsification, etc., and achieve long-term filling, uniform particle size and control, not easy to move and free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
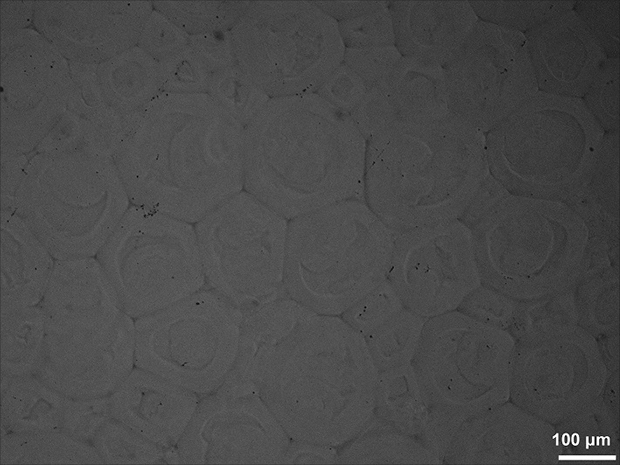
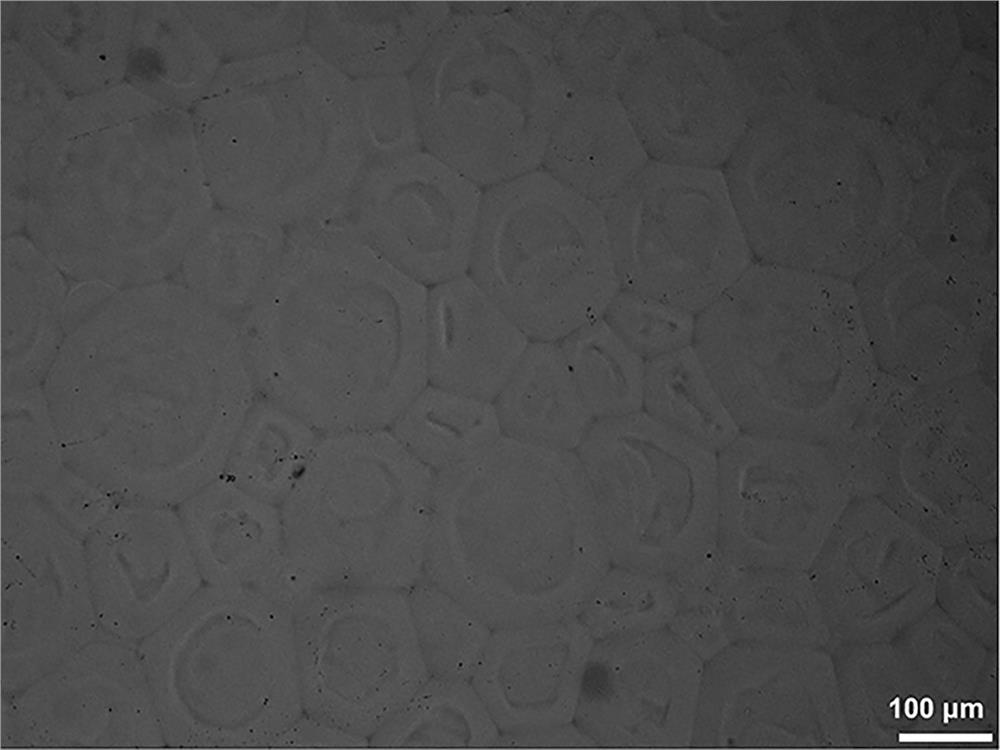
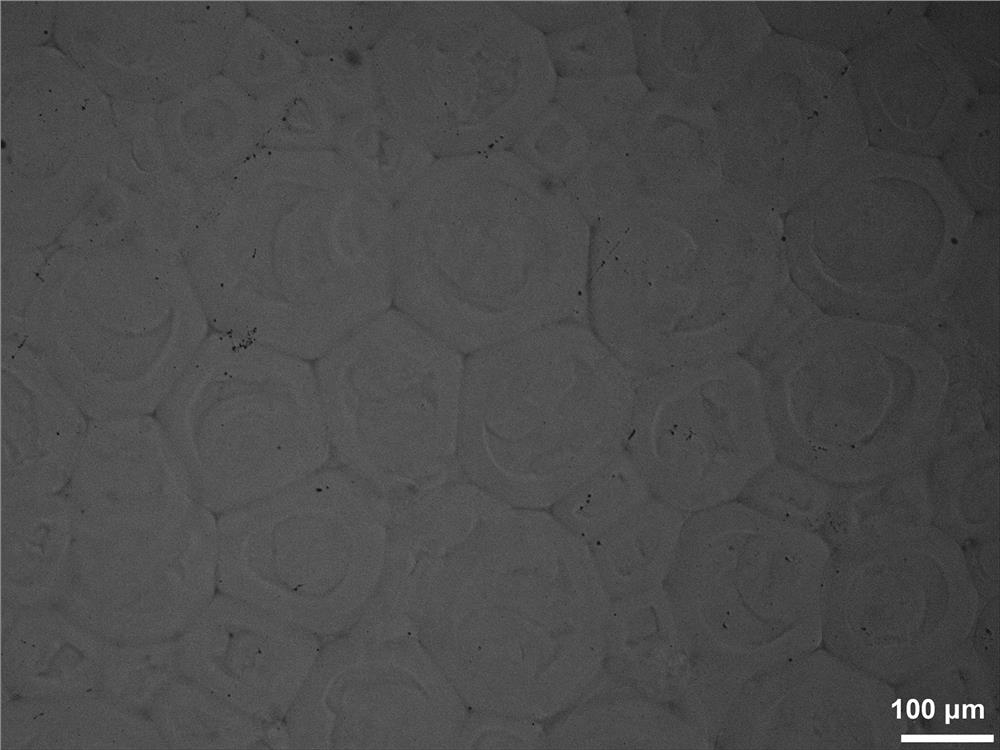
Image
Examples
Embodiment 1
[0033] (1) At room temperature, fully dissolve 20 g of dextran powder with a molecular weight of 40,000 g / mol in 200 mL of dimethyl sulfoxide solution, pass nitrogen gas for 30 minutes, add 0.4 g of dimethylaminopyridine and 1 g of N, N'-carbonyl di The imidazole-activated hydroxyethyl methacrylate continued to react in the closed system for 48 hours, and then stopped the reaction. The mixed solution was dialyzed by a regenerated cellulose dialysis bag with a cut-off molecular weight of 3000kDa, and the deionized water was replaced every 4-6 hours. After 7 days of dialysis, the polymerizable dextran was obtained by freeze-drying.
[0034] (2) 2 g of polymerizable dextran obtained in step (1) is fully dissolved in 15 mL of deionized water, and 45 mg of ammonium persulfate is added to obtain dispersed phase solution A; in 35 mL of cyclohexane solution, add ) Lecithin with a mass of 0.5%, mechanically stirred, and mixed uniformly to obtain a continuous phase solution B; the tempe...
Embodiment 2
[0037](1) At room temperature, fully dissolve 20 g of dextran powder with a molecular weight of 70,000 g / mol in 200 mL of dimethyl sulfoxide solution, pass nitrogen gas for 30 min, add 0.4 g of triethylamine and 1.5 g of glycidyl methacrylate, After continuing to react in the closed system for 48h, stop the reaction. The mixed solution was dialyzed by a regenerated cellulose dialysis bag with a cut-off molecular weight of 3000kDa, and the deionized water was replaced every 4-6 hours. After 7 days of dialysis, the polymerizable dextran was obtained by freeze-drying.
[0038] (2) Take 3 g of polymerizable dextran obtained in step (1) and fully dissolve it in 15 mL of deionized water, add 45 mg of ammonium persulfate to obtain dispersed phase solution A; in 35 mL of n-hexane solution, add relative to (A+B) Span 80 with a mass of 1.5%, mechanically stirred, and mixed uniformly to obtain the continuous phase solution B; set the temperature at 30°C, add the dispersed phase solution ...
Embodiment 3
[0041] (1) At room temperature, fully dissolve 20 g of dextran powder with a molecular weight of 100,000 g / mol in 200 mL of dimethyl sulfoxide solution, pass nitrogen gas for 30 minutes, add 0.4 g of triethylamine, 0.4 g of dimethylaminopyridine, 0.5 g of N , N'-carbonyldiimidazole-activated polylactic acid grafted hydroxyethyl methacrylate and 1.5g glycidyl methacrylate continued to react in a closed system for 48h, then stopped the reaction. Dialyze the mixed solution with regenerated cellulose dialysis bags with a cut-off molecular weight of 3000kDa, replace deionized water every 4-6 hours, and freeze-dry after 8 days to obtain polymerizable dextran.
[0042] (2) 1.5 g of polymerizable dextran obtained in step (1) was fully dissolved in 15 mL of deionized water, and 45 mg of ammonium persulfate was added to obtain dispersed phase solution A; in 30 mL of n-hexane and 5 mL of cyclohexane mixed solution, Add 1.5% Span 80 and 1.5% monoglyceride fatty acid ester relative to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com