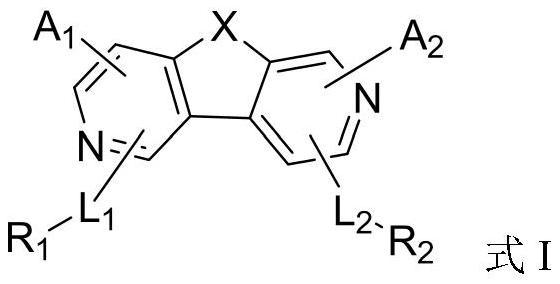
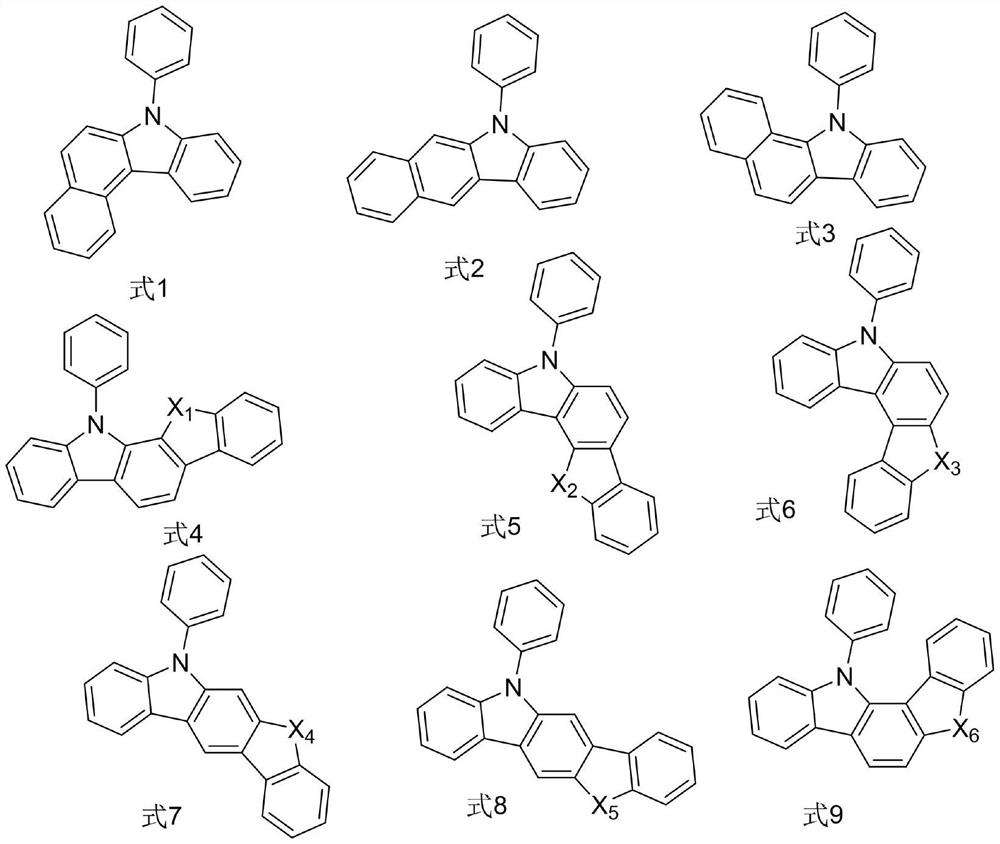
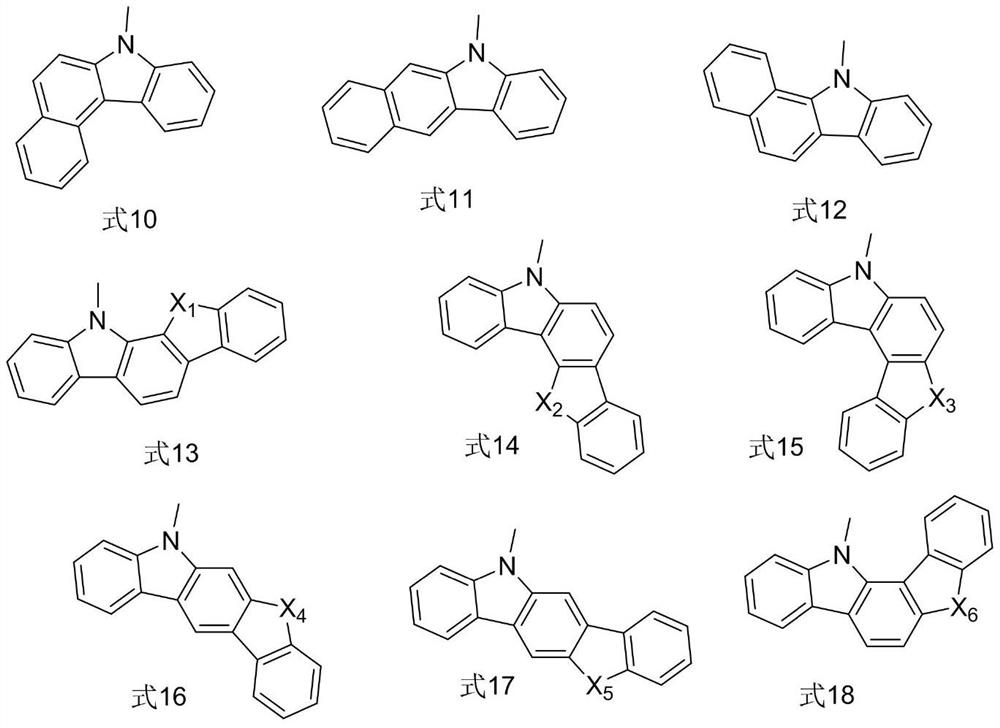
Organic compound and organic electroluminescent device
An organic compound and compound technology, applied in the field of organic electroluminescent devices, can solve the problems of high driving voltage and low current efficiency, and achieve the effects of reducing driving voltage, increasing steric resistance, and high energy level matching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0091] Preparation Example 1: Synthesis of Compound 7
[0092]
[0093]Synthesis of intermediate 7-1: In a 1L three-neck flask, add intermediate M1 (15g, 68.2mmol), N-phenyl-3-carbazole boronic acid (23.5g, 82mmol), 150ml isopropanol, 80ml water , anhydrous potassium carbonate (26.2g, 0.19mol) and bis (triphenylphosphine) palladium dichloride (0.5g, 0.7mmol), under the protection of nitrogen, heated and stirred, heated to reflux for 5h, and the reaction was reduced to Carry out liquid separation at room temperature, get the organic phase and add saturated sodium chloride and wash to neutrality, pass the organic phase through silica gel, the eluent is toluene, rinse with 500ml toluene, and the organic phase after the column is spin-dried under reduced pressure to obtain a white solid ( Yield: 75%).
[0094] Synthesis of Intermediate 7-2: In a 1L three-neck flask, add Intermediate 7-1 (21.4g, 50mmol), pyridine (10g, 0.126mol) and 250ml of dichloromethane, and start stirring....
preparation example 2
[0097] Preparation Example 2: Synthesis of Compound 22
[0098]
[0099] Synthesis of Intermediate 22-1: In a 1L three-necked flask, add 2-chloro-4,6-diphenylpyrimidine (15g, 56.4mmol), biborpinacol ester (17.1g, 67.2mmol), acetic acid Potassium (16.5 g, 0.168 mol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.41 g, 0.56 mmol) and 220 ml of 1,4-dioxane solvent , under the protection of nitrogen, heated and stirred, heated to reflux for 5.5h, after the reaction solution was lowered to room temperature, the reaction solution was spin-dried under reduced pressure to obtain the crude product, which was dissolved in chlorobenzene solvent, heated and stirred, heated to reflux, heated Pass through a silica gel column for decolorization, spin dry the filtrate under reduced pressure until there is a small amount of solvent, then add 200ml of ethanol for slurry, and recrystallize with toluene / ethanol to obtain a yellow solid (yield: 71%).
[0100] Synthesis of inter...
preparation example 3
[0104] Preparation Example 3: Synthesis of Compound 38
[0105]
[0106] Synthesis of Intermediate 38-1: The synthesis method was the same as that of Intermediate 22-1 to obtain a yellow-brown solid (yield: 76%).
[0107] Synthesis of Intermediate 38-2: The synthesis method was the same as that of Intermediate 7-1 to obtain an off-white solid (yield: 78%).
[0108] Synthesis of Intermediate 38-3: The synthesis method was the same as that of Intermediate 7-2 to obtain a white solid (yield: 90%).
[0109] Synthesis of compound 38: the synthesis method was the same as that of compound 7 to obtain a yellow solid (yield: 69%).
[0110] Mass spectrum: C43H26N6O, theoretical value: 642.22, measured value: 642.1. 1H-NMR (400MHz, CDCl3) (ppm) δ=7.10~7.21 (4H, m), 7.38~7.42 (1H, m), 7.48~7.53 (7H , m), 7.57~7.68 (2H, m), 7.92~7.96 (1H, d), 8.17~8.21 (2H, m), 8.27~8.29 (1H, s), 8.33~8.39 (4H, m), 8.47 ~8.51 (1H, d), 8.53~8.57 (1H, m), 8.59~8.61 (1H, m), 9.18~9.19 (1H, s).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap