Alcohol dehydrogenase and application thereof in synthesis of chiral heterocyclic alcohol
A technology of alcohol dehydrogenase and glucose dehydrogenase, which is applied in the fields of application, oxidoreductase, and microbial-based methods, can solve the problems of increased production cost, unfavorable production and application, and enzyme activity damage caused by excessive catalyst addition, and achieves The effect of mitigating the inhibitory effect of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
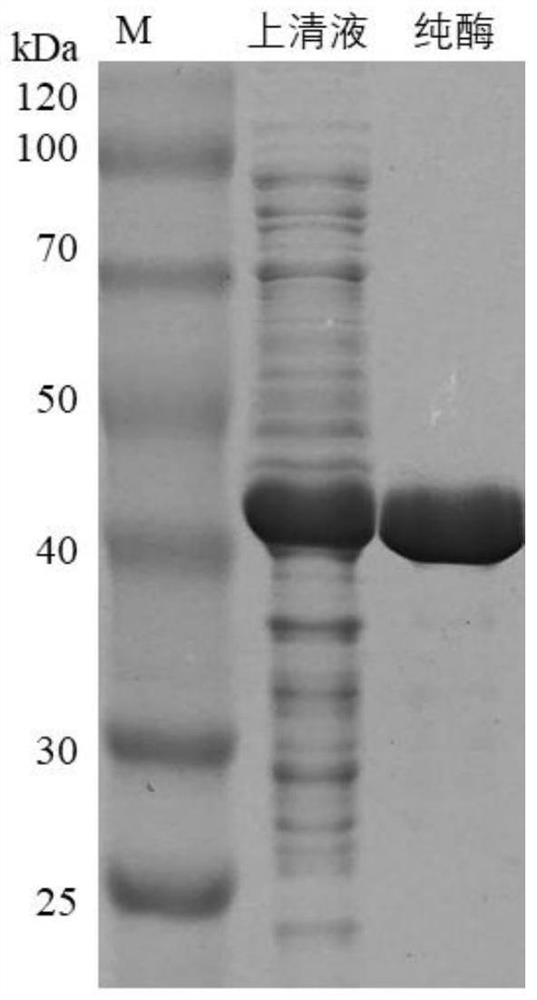
Image
Examples
Embodiment 1
[0085] Preparation of TB medium: Add 144g of yeast extract, 72g of peptone, 24g of glycerol, 4L of water, 10g of potassium dihydrogen phosphate, 12g of dipotassium hydrogen phosphate into a 5L fermenter, sterilize at 121°C for 20min, cool to 37°C, Get the corresponding TB medium.
[0086] Insert 60mL of recombinant Escherichia coli strains containing T7 promoter and expressing recombinant carbonyl reductase into TB medium, and then control the temperature at 37°C with aeration and stirring to activate the culture. After the OD600 reaches 6.0-7.0, lower the temperature to 25°C, add IPTG with a final concentration of 0.2mM, and then add carbon and nitrogen sources in an appropriate amount at an appropriate time, continue to control the temperature at 25°C, and obtain the corresponding fermentation broth after the fermentation is completed. The cells were collected by centrifugation and stored at -20°C for future use.
[0087] Take 100g of the bacteria obtained above, resuspend ...
Embodiment 2
[0089] In order to explore the substrate spectrum of CgADH, select heterocyclic ketone dihydro-3(2H)-furanone, tetrahydrothiophen-3-one, cyclohexanone, 4-ethylcyclohexanone, N-Boc-3-pyrrolidone , N-Boc-2-piperidone, N-Boc-3-piperidone, N-Boc-4-piperidone and other substrates were used to determine the activity and selectivity of CgADH to heterocyclic ketones. As shown in Table 1, CgADH is active for all substrates and has high stereoselectivity for substrates containing side chain substitutions.
[0090] Table 1: CgADH substrate profile analysis
[0091]
[0092]
[0093] Note: N.A.: notavailable (none)
Embodiment 3
[0094] Embodiment 3: the influence of reaction pH on the synthetic R-NBHP of alcohol dehydrogenase CgADH
[0095] Three pHs (pH5.0, pH6.0, pH7.0) were chosen to explore the optimal reaction pH of alcohol dehydrogenase CgADH. Add 50 mg of CgADH and 40 mg of BmGDH lyophilized enzyme powder to a 20 mL reaction system, which contains 0.4 g of substrate and 0.6 g of glucose. During the reaction process, firstly add lyophilized enzyme powder and PBS7.0 or PBS6.0 buffer solution and stir evenly, then add substrate and glucose at one time. It can be seen from Table 2 that the optimal reaction pH of CgADH is 6.0.
[0096] Table 2 Optimization of reaction pH
[0097]
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com