Human serum albumin nano-drug based on metabolic checkpoints as well as preparation method and application of human serum albumin nano-drug
A technology of human serum albumin and nano-drugs, applied in the field of biomedicine, can solve the problems of side effects of weak anti-tumor drugs, achieve the effect of improving treatment efficiency, improving stability, and overcoming toxicity challenges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the double drug coupling prodrug molecule comprises: Fmoc group protected 1-methyl tryptophan (1-MT) and isopropyl-DON are coupled through an amide bond, and the obtained coupling The molecule is detached from the Fmoc group through a hydrolysis reaction to obtain a double-drug coupling prodrug molecule, which specifically includes the following steps:
[0043] (1) Isopropyl-DON and 9-fluorenylmethoxycarbonyl-1-methyltryptophan in benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate In the presence of (HBTU) and diisopropylethylamine, coupled to generate Fmoc-protected IDOi-DON;
[0044] (2) The Fmoc-protected IDOi-DON obtained in step (1) reacted overnight under the action of piperidine to remove the Fmoc protecting group to obtain the target product IDOi-DON;
[0045] The present invention also provides a method for preparing human serum albumin nano-medicines (IDNPs) based on the Tie checkpoint-immune activator prodrug, using the ...
Embodiment 1
[0051] Example 1 Synthesis of IDOi-DON Protected by 9-Fluorenylmethoxycarbonyl
[0052] The specific preparation method is as follows:
[0053] Weigh 880 mg of 9-fluorenylmethoxycarbonyl-1-methyl tryptophan (2.06 mmol, 1.1 equivalents) and 854 mg of HBTU (2.25 mmol, 1.2 equivalents) and dissolve in 14 mL of dry N,N-dimethyl Formamide (DMF), then sequentially added 980 μL of diisopropylethylamine (727 mg, 5.63 mmol, 3 equivalents) and isopropyl-DON (400 mg, 1.88 mmol, 1 equivalent) in dry DMF solution, in an inert gas atmosphere Stirring was continued for 4 hours.
[0054] DMF was removed by rotary evaporation, 100 mL of dichloromethane (DCM) was added, and the resulting organic solutions were washed with 100 mL of saturated sodium bicarbonate (NaHCO 3 ) solution, water, 1mol / L hydrochloric acid (HCl), water and saturated sodium chloride (NaCl) solution, and dried with anhydrous magnesium sulfate. DCM was evaporated and column chromatography (separation solvent: dichlorometh...
Embodiment 2
[0056] Example 2 Preparation of IDOi-DON by removing the carbonyl group of 9-fluorenylmethyloxy group
[0057] Weigh 9-fluorenylmethoxycarbonyl-IDOi-DON (900 mg, 2.07 mmol, 1 equiv) and dissolve in 10 mL of dry DCM, slowly add piperidine (514 μL, 5.17 mmol, 2.5 equiv) dropwise, and then continue at Stir at room temperature for 4 hours.
[0058] The organic solvent was removed by rotary evaporation and vacuum pump, and purified by column chromatography (separation solvent: chloroform / methanol = 30 / 1) to obtain the yellow product IDOi-DON. The synthetic route is shown below.
[0059]
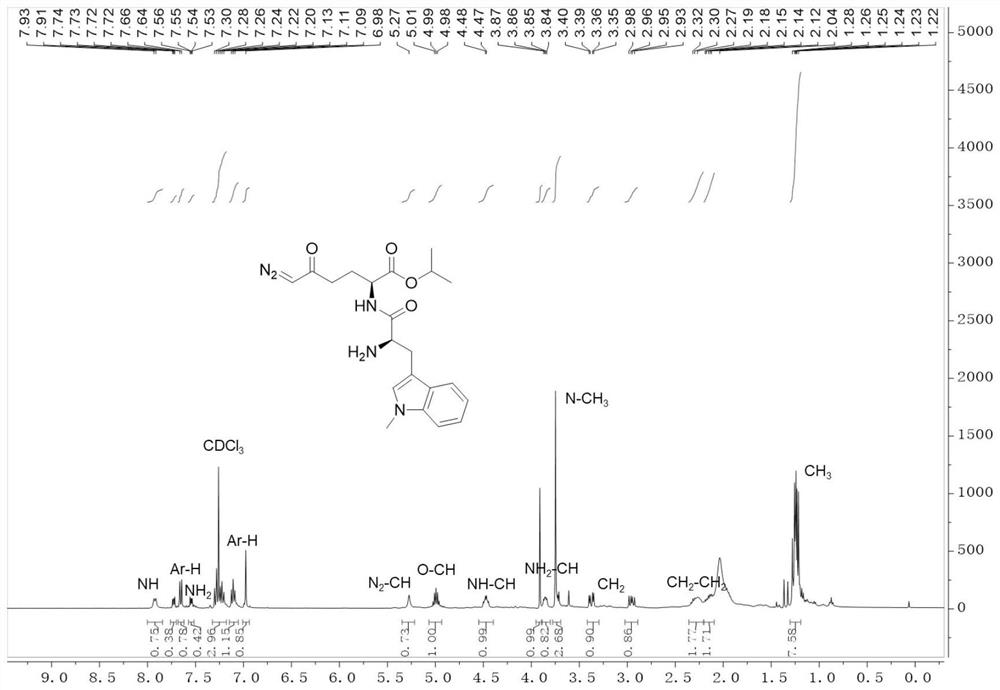
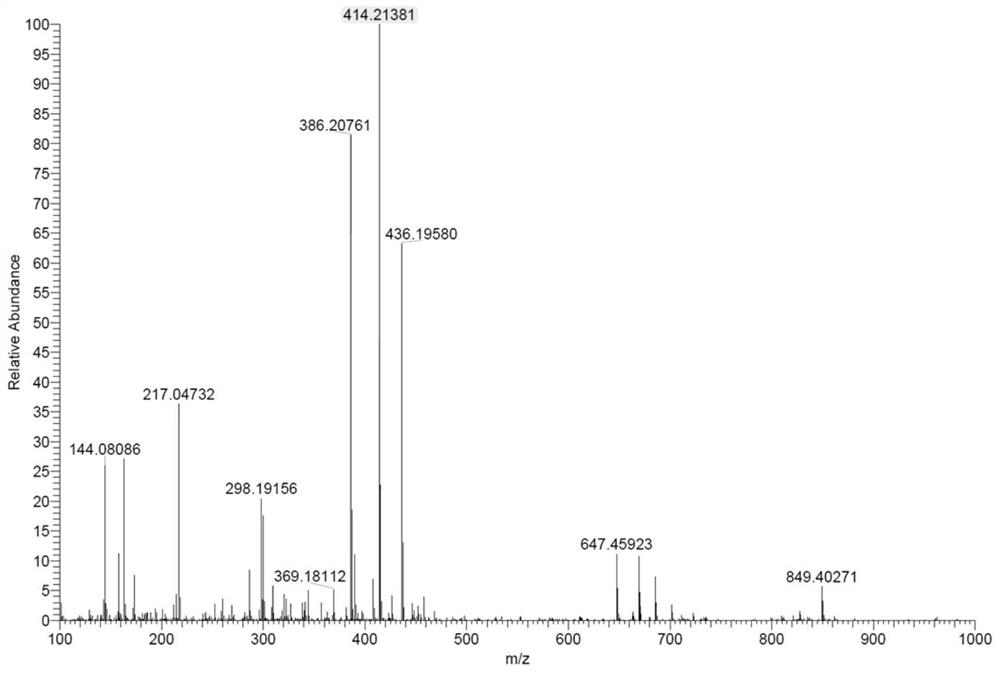
[0060] Characterize the obtained IDOi-DON, its H NMR spectrum is as follows figure 1 As shown, the peak at ~3.7ppm belongs to the -CH on the indole ring 3 , the ~1.3ppm peak is attributed to the two -CH of isopropyl 3 . The mass spectrum of IDOi-DON as figure 2 As shown, wherein 414m / z belongs to the protonation peak of the prepared IDOi-DON.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com