Synthesis method of flomoxef intermediate
A fluoxetin and intermediate technology, applied in the field of drug synthesis, can solve the problems of unfavorable environmental protection, operator safety protection, unfavorable industrial production, long steps of intermediate 1, etc., and achieve short reaction route, short route and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
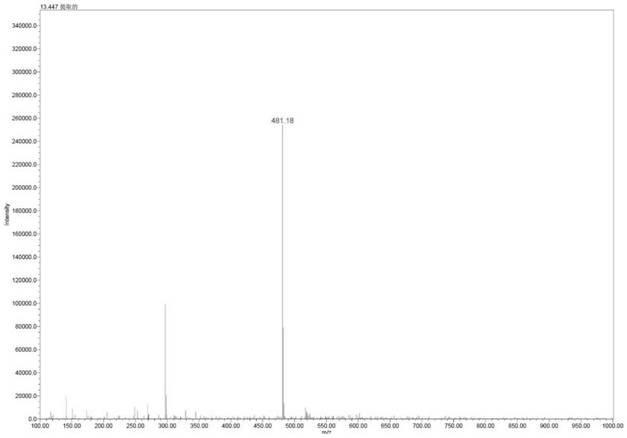
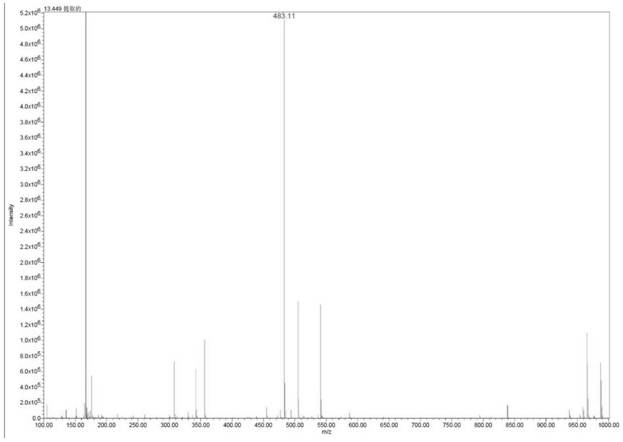
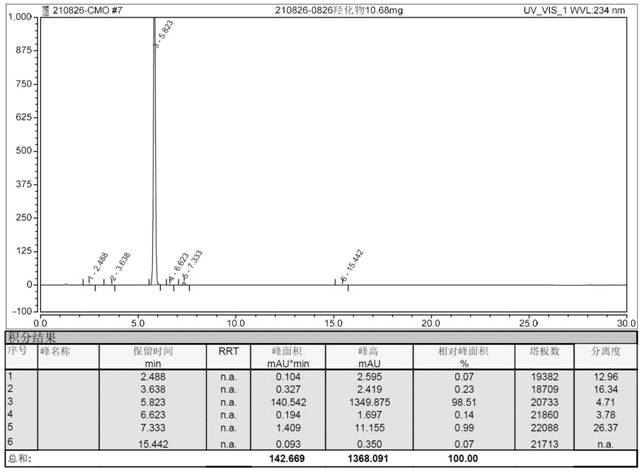
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 intermediate 1
[0035] Add 40mL of selenium dioxide (87.2mg, 0.784mmol) aqueous solution to 100mL dry round bottom flask, cool to 0°C, slowly add 0.72mL tert-butyl hydroperoxide (5.88mmol) 70% aqueous solution, stir at 0°C 30 minutes. Then 10mL of dichloromethane solution containing compound 3 (1.82g, 3.92mmol) was added dropwise. After the dropwise addition was completed, the reaction was carried out at room temperature. After the reaction was complete, 1.5M aqueous sodium hydroxide solution was added to the reaction system to adjust the pH to neutral. Quench the reaction, then add 20mL of dichloromethane for extraction, separate the organic layer, dry over anhydrous magnesium sulfate, concentrate under reduced pressure to remove the dichloromethane to obtain a yellow oil, add 12mL of methanol, crystallize at 0-10°C, filter and dry , 1.44 g of the target product was obtained, the yield was 76%, and the HPLC purity was 98.51%.
[0036]...
Embodiment 2
[0037] The preparation of embodiment 2 intermediate 1
[0038] Add 30 mL of selenium dioxide (130 mg, 1.18 mmol) in dichloromethane solution to a 100 mL dry round bottom flask, cool to 0° C., slowly add 1 mL of aqueous benzoic acid peroxide (0.3 g, 6.48 mmol), and stir at 20° C. for 30 minute. Then add dropwise a dichloromethane solution containing 10mL of compound 3 (1.82g, 3.92mmol). After the dropwise addition, react at room temperature. After the reaction is complete, add 1M aqueous sodium carbonate solution to the reaction system, adjust the pH to neutral, and quench the reaction. , and then add 15mL of dichloromethane for extraction, separate the organic layer, dry over anhydrous magnesium sulfate, concentrate under reduced pressure to remove dichloromethane to obtain a yellow oil, add 15mL of ethanol, crystallize at 0-10°C, filter, and dry to obtain the target Product 1.38g, yield 73%.
Embodiment 3
[0039] The preparation of embodiment 3 intermediate 1
[0040]Add 20 mL of selenium dioxide (120 mg, 1.08 mmol) in ethyl acetate to a 100 mL dry round bottom flask, cool to 0°C, slowly add 0.5mL of acetic acid (0.2g, 3.3mmol) aqueous solution, and stir at 30°C for 30 minutes. Then add dropwise an ethyl acetate solution containing 5mL of compound 3 (0.91g, 1.96mmol). After the dropwise addition, react at room temperature. After the reaction is complete, add 1M potassium carbonate aqueous solution to the reaction system, adjust the pH to neutral, and quench the reaction. , then add 10 mL of dichloromethane for extraction, separate the organic layer, dry over anhydrous magnesium sulfate, concentrate under reduced pressure to remove the dichloromethane to obtain a yellow oil, add 7 mL of methanol / acetone (2:1), and crystallize at 0-10°C , filtered, and dried to obtain 0.66 g of the target product, with a yield of 70%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com