Preparation and application of injectable bone repair material capable of activating endogenous TGF beta1 in situ
An injection type, bone repair technology, applied in the direction of prosthesis, drug delivery, tissue regeneration, etc., can solve problems affecting the process of bone repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Injectable Bone Repair Material That Can Activate Endogenous TGFβ1 in Situ
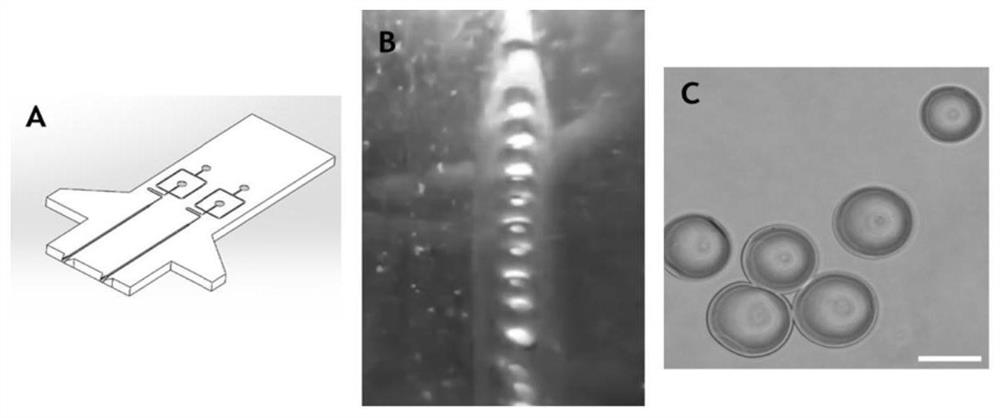
[0039] The hydrogel material is divided into two parts: matrix and gelatin microspheres. The gelatin microspheres are prepared in a microfluidic manner. In order to ensure the injectability of the final hydrogel material, the diameter of the prepared gelatin microspheres needs to be controlled at about 120 μm.
[0040] (1) Preparation of Type A gelatin microspheres: The core component of the microfluidic system is the microfluidic chip. The chip is designed with SolidworksCAD software and then CNC-cut. It is made of PMMA resin with a thickness of 3 mm and an internal channel width of 150 μm. The water phase was prepared with 5% Type A gelatin solution, the oil phase was mineral oil containing 20wt% surfactant Span80, and the injection speeds of the oil phase and the water phase were 18.8ul / min and 3.08ul / min, respectively. The collection device was placed in a water ...
Embodiment 2
[0045] Example 2 Application of Injectable Bone Repair Materials That Can Activate Endogenous TGFβ1 in Situ
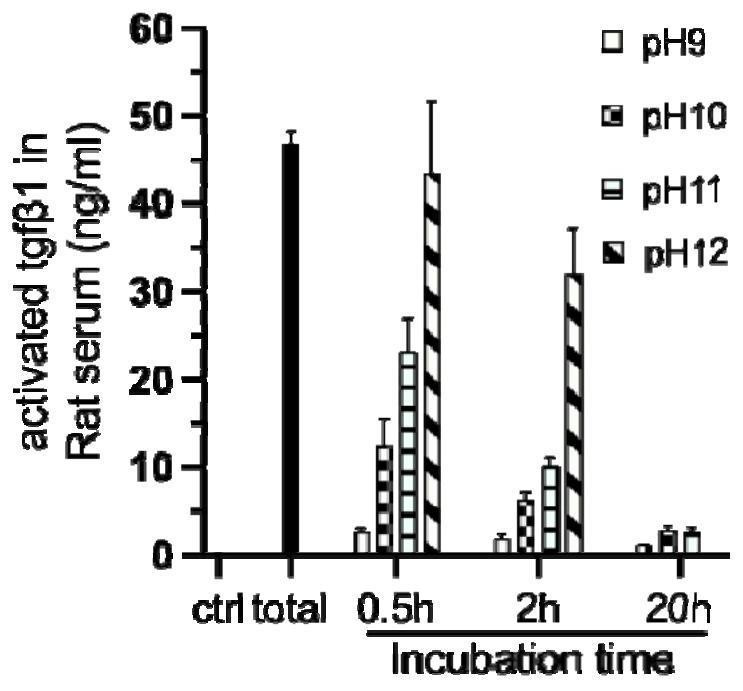
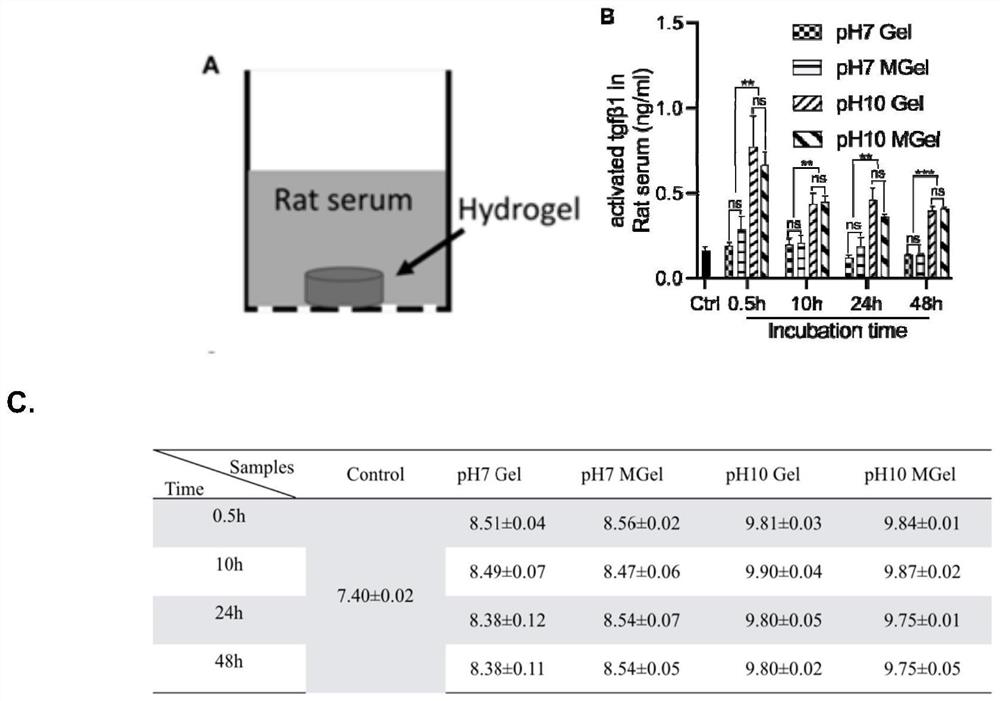
[0046] Firstly, a SD rat skull defect model was constructed. Anesthetized by intraperitoneal injection of 2% pentobarbital sodium, fixed the head of the rat, prepared the skin, disinfected with 2% povidone iodine, cut the skin and muscular layer 5-8mm in the middle of the cranial suture, and peeled off the periosteum to expose the bone surface. A circular bone defect was made on both sides of the cranial suture with a trephine drill with an outer diameter of 5 mm, and pH7 PEI-modified gelatin-based microsphere hydrogel (pH7 Mgel) and pH10 PEI-modified gelatin-based microsphere hydrogel were injected into the defect area (ph10 Mgel), the muscle layer was sutured with 5-0 absorbable suture, and the skin layer was sutured with 5-0 silk suture. After the operation, the rats were placed on a 37°C heat preservation pad until the rats regained consciousness, and they were fe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com