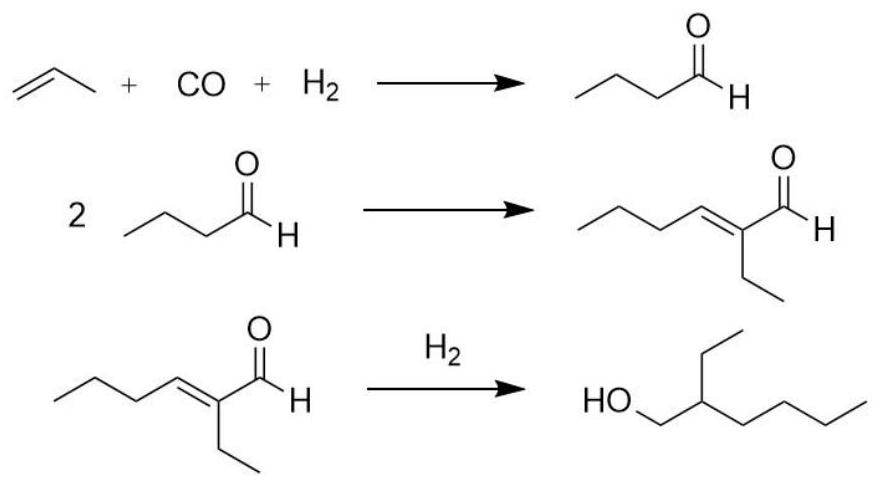
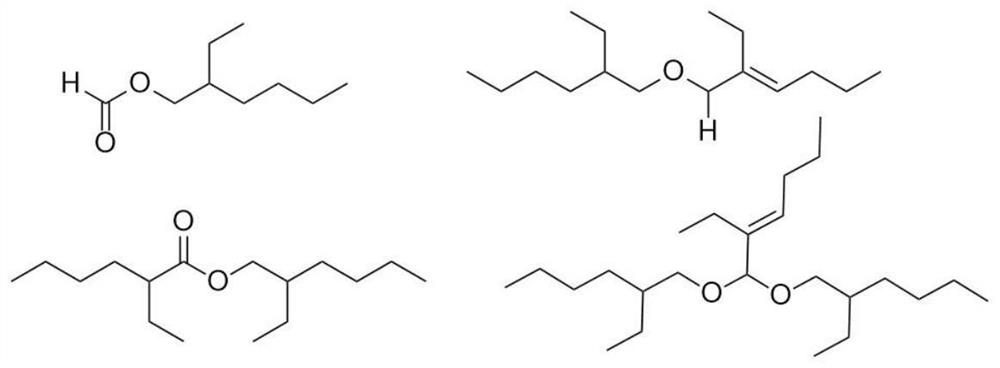
Method for promoting hydrolysis of isooctyl isocaprylate and isooctyl formate
A technology of isooctyl ester and isooctanoic acid, which is applied in the field of recycling of by-products of fine chemical isooctyl alcohol, can solve the problem that heavy components cannot be fully utilized, and achieve the effects of low cost, high hydrolysis activity and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 catalyst
[0033] 0.5gNbCl 5 Add to 10 ml of 1mol / L ammonia water, stir and dissolve to form a colloid. Add 5.0 g CeO to the formed colloid 2 , at 50°C, after stirring for 4 hours, after suction filtration and water washing three times, drying at 100°C, the cerium oxide solid adsorbed with hydrated niobium pentoxide was obtained, and finally activated by roasting at 900°C under nitrogen atmosphere to obtain A small amount of composite oxide support of niobium pentoxide (Nb 2 o 5 @CeO 2 ).
[0034] Dissolve 0.5g of butyl stannoic acid in 5ml of acetone, and evenly add dropwise to 4.5g of the synthesized Nb 2 o 5 @CeO 2 On the carrier, dry at 60° C. for 12 hours to obtain catalyst A, and the loading amount of butyl stannoic acid is 10%.
[0035] 0.5g Ce(NO 3 ) 2 Add to 6 ml of 1mol / L aqueous oxalic acid solution, add 5.0 g of Nb to the resulting solution 2 o 5 , stirred at 40°C for 6 hours, then added 4 ml of 25% ammonia water ...
Embodiment 2
[0038] In a 1L autoclave, add isooctyl isooctanoate (274g, 1mol) and 200g of water, and add the above-mentioned catalyst A, 2.7g. The reaction kettle was stirred at 120°C, protected by nitrogen gas, and maintained at a slight positive pressure. Keeping the reaction temperature, after reacting for 8 hours, a sample was taken to analyze the composition of the product by gas chromatography. It was found that the hydrolysis conversion rate of isooctyl isooctanoate was 99%, and the hydrolyzed products were isooctanoic acid and isooctyl alcohol.
Embodiment 3
[0040] In a 1L autoclave, add isooctyl isooctanoate (274g, 1mol) and 200g of water, and add the above-mentioned catalyst B, 2.7g. The reaction kettle was stirred at 120°C, protected by nitrogen gas, and maintained at a slight positive pressure. Keeping the reaction temperature, after reacting for 8 hours, a sample was taken to analyze the composition of the product by gas chromatography. It was found that the hydrolysis conversion rate of isooctyl isooctanoate was 98%, and the hydrolyzed products were isooctanoic acid and isooctyl alcohol. After the reaction, the catalyst B was separated by filtration, the filtrate was allowed to stand for stratification, and the water was removed, and it was pickled with dilute sulfuric acid with a concentration of 9.0%, and the organic phase was collected to obtain a hydrolyzed crude product. After the catalyst B was washed with water in equal volume three times, the °C under a nitrogen atmosphere for 4 hours to obtain recovered catalyst B f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com