Preparation method of p-dodecylphenol
A technology of dodecylphenol and alkylation, applied in the field of preparation of p-dodecylphenol, can solve the problems of unfavorable p-dodecylphenol production, long synthesis distance, increased production cost, etc. Healthy and sustainable development, high content of para-structure, and the effect of promoting development and progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
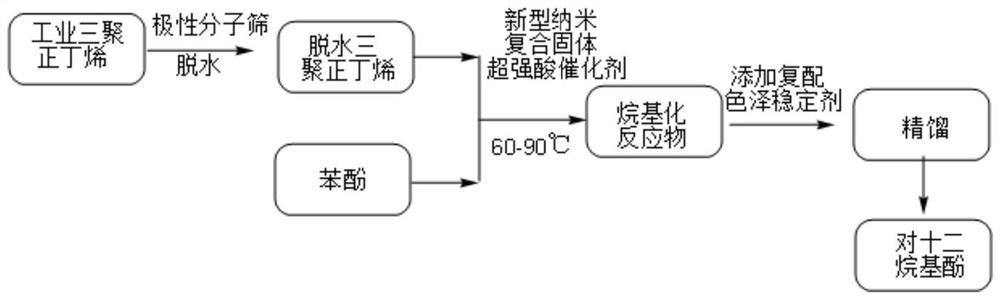
[0035] Such as figure 1 Shown, the first aspect of the present invention provides a kind of preparation method of dodecylphenol, comprising:
[0036] Dehydration: adding olefin raw materials to metal hydroxide-loaded molecular sieves for dehydration to obtain dehydrated olefins;
[0037] Alkylation: add dehydrated olefin and phenol with a molar ratio of 1: (3-5) to the catalyst, and react at 60-120°C to obtain an alkylated crude product;
[0038] Rectification: rectify the crude alkylation product to obtain p-dodecylphenol product.
[0039] dehydration
[0040] In one embodiment, the olefin raw material of the present invention is selected from one of n-dodecene, halogenated dodecane, n-dodecyl alcohol, tetrapropylene, trimerized isobutene, trimerized n-butene, preferably The base is tripoly-n-butene.
[0041] A small amount of bound water and free water in trimeric n-butene in industry, because the catalyst used in the present invention is sensitive to water, it is necess...
Embodiment 1-1~1-6、2-1~2-7
[0078] Embodiment 1-1~1-6, 2-1~2-7: Dehydration
[0079] Examples 1-1 to 1-6 and Examples 2-1 to 2-7 provide methods for the dehydration of trimeric n-butene: adding metal hydroxide-supported molecular sieves to olefin raw materials to obtain dehydrated olefins; the metal hydroxide The preparation method of the metal hydroxide-loaded molecular sieve is as follows: adding sodium hydroxide to water, adding 13X molecular sieve for adsorption, drying, and roasting to obtain the metal hydroxide-loaded molecular sieve.
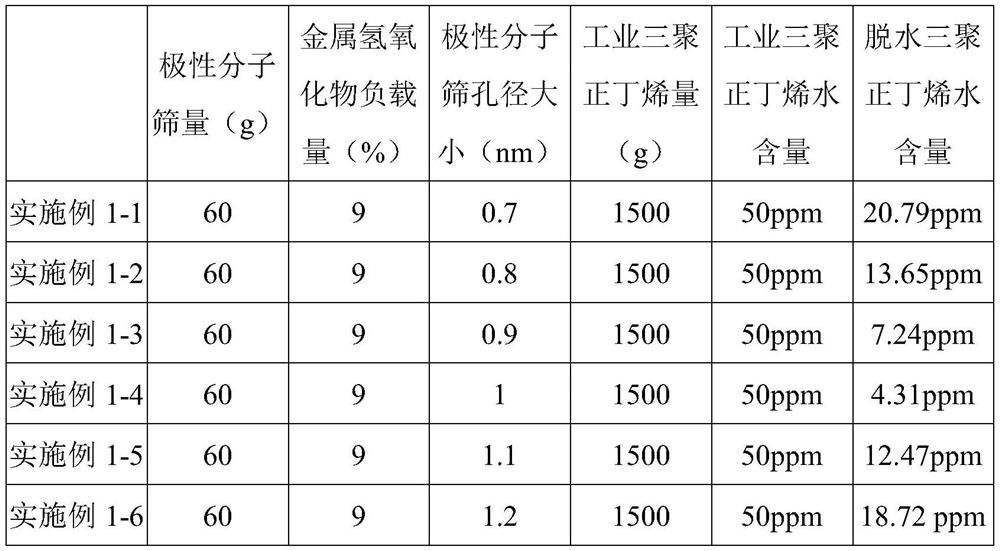
[0080] Examples 1-1 to 1-6 See Table 1 for the dehydration test results of the molecular sieve pore size of polar molecular sieves.
[0081] Table 1
[0082]
[0083] Examples 2-1 to 2-7 The dehydration test results of testing the metal hydroxide loading capacity of polar molecular sieves are shown in Table 2.
[0084] Table 2
[0085]
Embodiment 3-1~3-6、4-1~4-6、5-1~5-7
[0086] Embodiment 3-1~3-6, 4-1~4-6, 5-1~5-7: Alkylation
[0087] Embodiments 3-1 to 3-6, 4-1 to 4-6, and 5-1 to 5-7 provide dehydrated olefin and phenol alkylation methods, including:
[0088] Put the catalyst into the reactor, pass nitrogen gas, activate at 210°C for 4 hours, add dehydrated olefin and phenol with a molar ratio of (1:4), and react to obtain the crude alkylated product. The dehydrated olefin is the dehydrated olefin provided in Example 2-5.
[0089] The preparation method of described catalyst comprises: ZrO(NO 3 ) 2 2H 2 O added to water, adding nano ZrO 2 , adjust the pH to 8-9, precipitate and dry, add sulfuric acid solution at 75°C and mix for 2 hours, filter, dry, and roast at 450°C for 4 hours to obtain metal compound-loaded nanocarriers.
[0090] The crude alkylated products obtained in Examples 3-1~3-6, 4-1~4-6, 5-1~5-7 were analyzed using SP-2100 type chromatography, and the analytical column model of the chromatography was HP-PONA Chromatograp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com