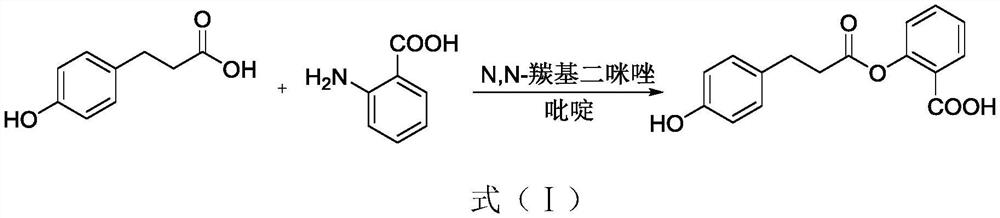
Preparation method of dihydrooat alkaloid
A technology of oat alkaloids and dihydrogen, which is applied in the field of preparation of dihydrooat alkaloids, can solve the problems of being unsuitable for industrial production, long synthesis route, and large environmental pollution, and achieves convenience for large-scale production, short preparation process steps, The effect of low solvent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of dihydrooat alkaloids, comprising the steps of:
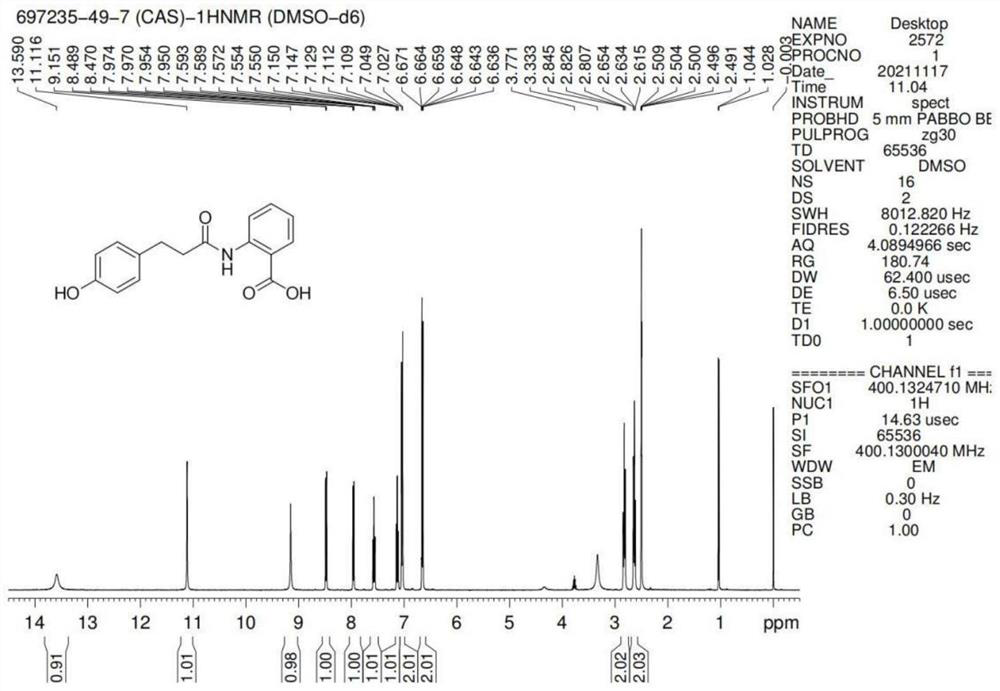
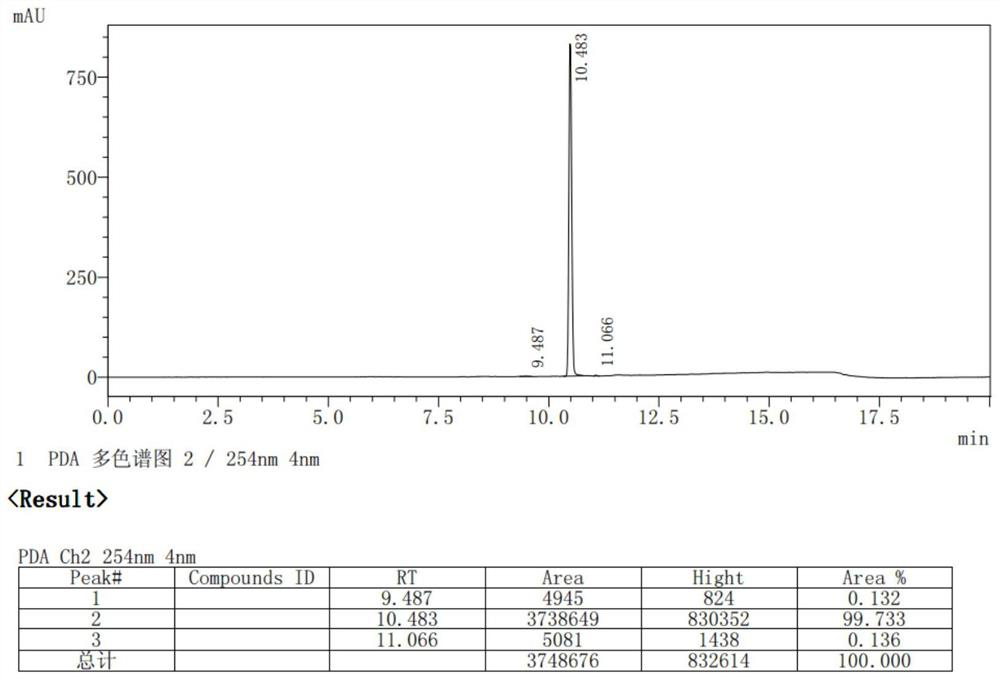
[0039] S1. Preparation of methyl p-hydroxyphenylpropionate: In a 250mL three-neck flask, add p-hydroxyphenylpropionic acid (50g, 1.0eq), 150mL of methanol and 5g of concentrated sulfuric acid in sequence, heat up and reflux for 6-8 hours, and monitor the end of the reaction by TLC Finally, excess methanol was removed to obtain a brown oil, 100 mL of dichloromethane was added to the oil, and then 50 mL of water was added to separate the organic phase, which was then dried with saturated sodium bicarbonate solution, anhydrous magnesium sulfate, and concentrated 53 g of oil was obtained, the HPLC purity was greater than 98%, and the oil was recrystallized from an ethanol / water mixed solvent to obtain 48 g of off-white crystal powder (methyl p-hydroxyphenylpropionate), with a melting point of 38-39° C. and a yield of 89.2%;
[0040] S2. Preparation of crude dihydrooat alkaloids: In a 250mL three-necked...
Embodiment 2
[0046] A preparation method of dihydrooat alkaloids, comprising the steps of:
[0047] S1. Preparation of ethyl p-hydroxyphenylpropionate: In a 250mL three-necked flask, add p-hydroxyphenylpropionic acid (50g, 1.0eq), 150mL of absolute ethanol and 5g of concentrated sulfuric acid in sequence, and heat up and reflux for 6-8h, monitor by TLC After the reaction, excess ethanol was removed to obtain a brown oil, which was extracted by adding petroleum ether, followed by drying with saturated sodium bicarbonate solution, anhydrous magnesium sulfate, and concentrating to obtain 53.1 g of oil with an HPLC purity of 99.6 %, yield 91.5%;
[0048] S2, the preparation of dihydrooat alkaloid crude product: in 250mL there-necked flask, add ethyl p-hydroxyphenylpropionate (50g, 1.0eq), sulfolane (100mL, 2V), anthranilic acid (35g, 1.0eq) and Concentrated sulfuric acid (5g, 0.17X), heat up to 130-140°C, keep warm for 6-8h; then cool down to below 70°C, slowly add 200-400mL of water, and the...
Embodiment 3
[0051] A preparation method of dihydrooat alkaloids, comprising the steps of:
[0052] S1. Preparation of methyl p-hydroxyphenylpropionate: In a 250mL three-necked flask, add p-hydroxyphenylpropionic acid (50g, 1.0eq), 100mL of methanol and 4.75g of concentrated sulfuric acid in sequence, heat and reflux for 6-8 hours, and monitor the reaction by TLC After the end, remove excess methanol to obtain a brown oil, add 100mL of dichloromethane to the oil, then add 50mL of water, separate the organic phase, and then dry the organic phase with saturated sodium bicarbonate solution, anhydrous magnesium sulfate, and concentrate Finally, 53g of oily matter was obtained, and the HPLC purity was greater than 98%. The oily matter was recrystallized from an ethanol / water mixed solvent to obtain 48g of off-white crystal powder (methyl p-hydroxyphenylpropionate), with a melting point of 38-39°C and a yield of 89.2%. ;
[0053] S2. Preparation of crude dihydrooat alkaloids: In a 250mL three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com