Clinical biochemical composite quality control product and preparation method thereof
A quality control, biochemical technology, applied in the preparation, sampling, and instrumentation of test samples, which can solve the problem of inability to clinical use, provide greater convenience, fail to solve product stability, and increase the risk of enzyme damage, etc. problems, to achieve the effect of easy reconstitution and mixing, ensuring the correctness of the test and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
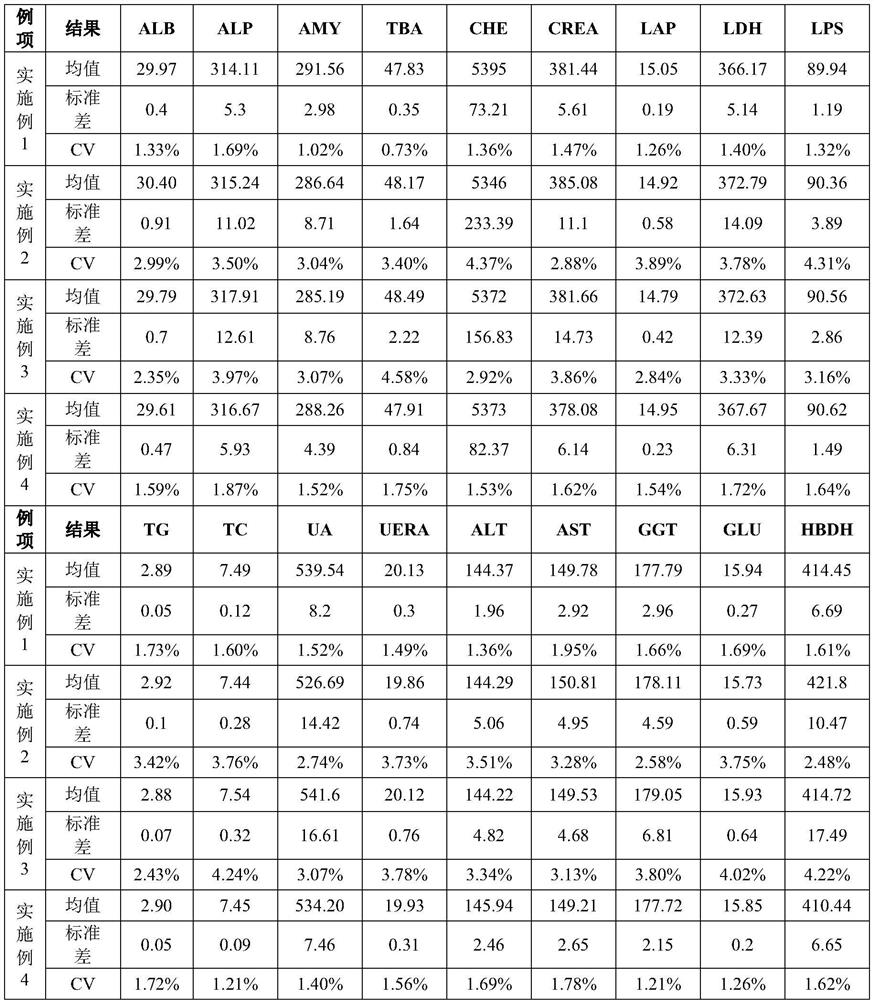
Embodiment 1
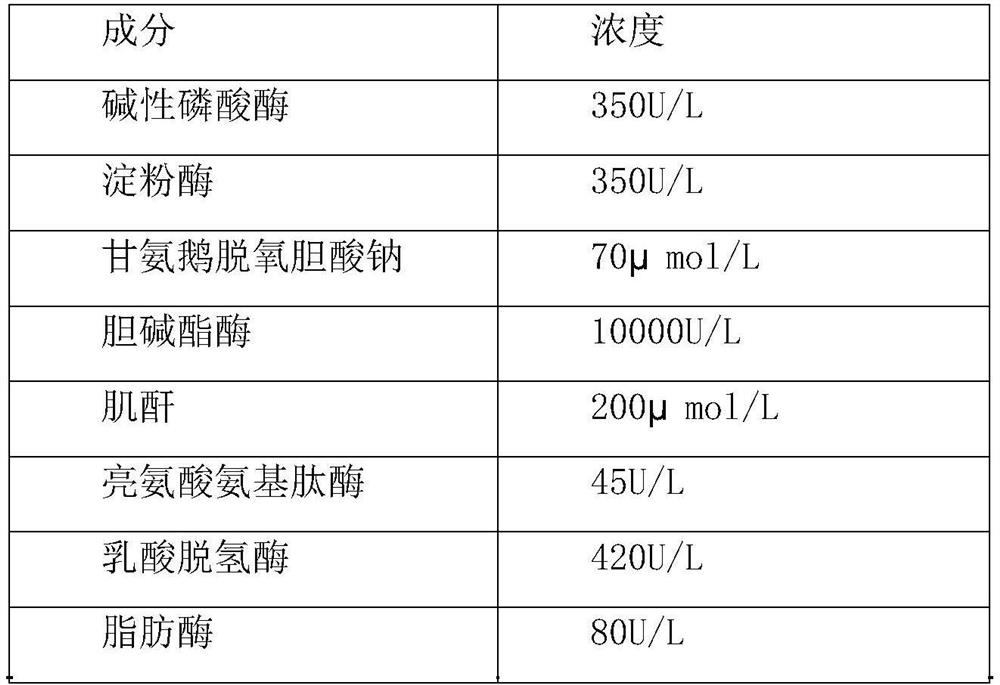
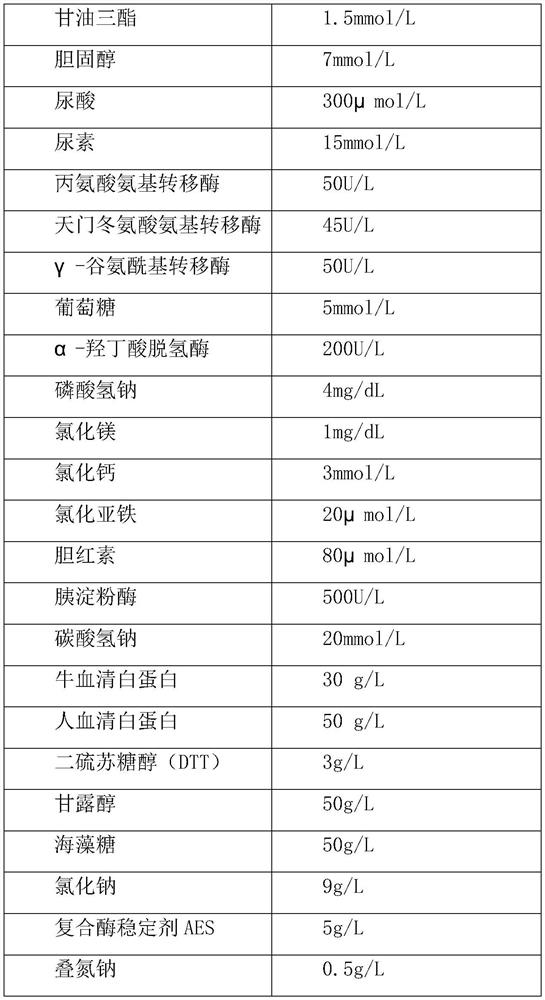
[0033] A clinical biochemical composite quality control product, including testing project raw materials, matrix liquid and protective agent, the specific formula is as follows:
[0034]
[0035]
[0036] The specific preparation method of the clinical biochemical composite quality control product of the present invention comprises the following steps:
[0037] Step 1. Add distilled water to the preparation container according to the preparation amount of the above formula, and set aside.
[0038] Step 2. Add bovine serum albumin and human serum albumin under stirring. After adding the materials, stir at 400 r / min for 30 minutes until completely dissolved.
[0039] Step 3. After the above is completely dissolved, use a filter membrane with a pore size of 0.22 μm to filter into another clean preparation container;
[0040] Step 4. Add dithiothreitol, mannose, trehalose, sodium chloride, compound enzyme stabilizer AES and sodium azide into the above solution in turn, and ...
Embodiment 2
[0053] The difference between this example and Example 1 is that human serum albumin is not added to the formula.
Embodiment 3
[0055] The difference between this example and Example 1 is that the compound enzyme stabilizer AES is not added to the formula.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com