Preparation method of L-cystine with high light transmittance
A technology with high light transmittance and cystine, applied in the preparation of organic compounds, chemical instruments and methods, hydrogenated polysulfide/polysulfide preparation, etc., can solve the problems that cannot meet the needs of industrial production, are not suitable for industrial production, reaction Long time and other problems, to avoid the reduction of light transmittance, less side reactions, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preparation method of high transmittance L-cystine, the specific steps are as follows:
[0036] S1. add L-cysteine hydrochloride monohydrate into purified water, stir until dissolved, and then adjust pH with inorganic base stirring to obtain solution A;
[0037] S2. add oxidizing agent in the solution A that S1 obtains, react to finish obtaining solution B;
[0038] S3. the solution B obtained by S2 is cooled and stirred for crystallization, filtered to obtain the L-cystine crude product;
[0039] S4. The L-cystine crude product obtained in S3 is washed with purified water, filter cake, and air-dried to obtain the L-cystine finished product.
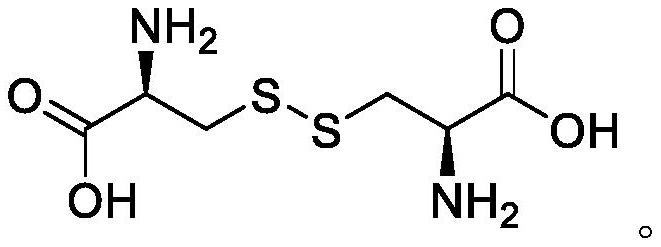
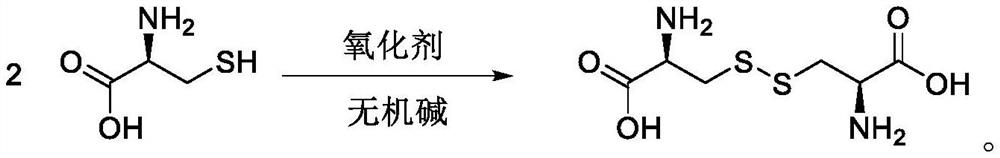
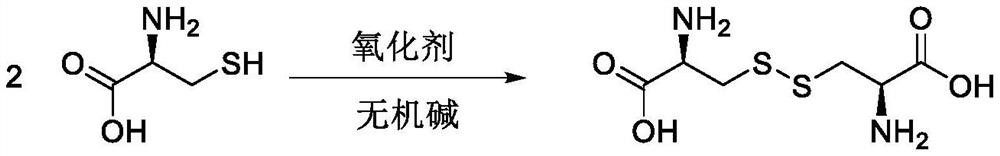
[0040] The reaction equation is as follows:
[0041]
[0042] Further, the inorganic bases in S1 include but are not limited to one or more of sodium hydroxide, potassium hydroxide, ammonia water, sodium carbonate, and sodium bicarbonate.
[0043] Further, in the S1, adjust the pH to 7-8 to stop stirring.
[0044] Furth...
Embodiment 1
[0052] Add 50g cysteine hydrochloride monohydrate and 250g purified water to a 500ml brown four-necked bottle, stir until all dissolved, cool to 5°C, add ammonia water dropwise to adjust pH=8.1, stop stirring, and keep the temperature at 5- 10°C, dropwise add 72.8g of 20% hydrogen peroxide, naturally heat up to room temperature, keep the temperature for 4h, cool to 5-10°C for crystallization for 2h, from the start of the reaction to the end of crystallization, stir every 2h, filter, 20g purified water The filter cake was washed and dried to constant weight at 55-65°C to obtain 32.5 g of L-cystine crude product.
Embodiment 2
[0054]Add 50g of cysteine hydrochloride monohydrate and 300g of purified water to a 500ml brown four-necked flask, stir until all dissolved, cool to 5°C, dropwise add 5% sodium hydroxide solution to adjust pH=7.5, stop stirring, Keep the temperature at 5-10°C, add 25.13g of DMSO dropwise, heat up to room temperature naturally, keep the reaction for 4h, the reaction is over, cool down to 5-10°C for crystallization for 3h, from the start of the reaction to the end of the crystallization, stir every 2h, Filter, wash the filter cake with 20 g of purified water, and dry to constant weight at 55-65 °C to obtain 31.77 g of crude cystine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com