Ester pentaacetyl geniposide derivative obtained through esterification reaction as well as preparation method and application of ester pentaacetyl geniposide derivative
A technology of pentaacetyl geniposide and geniposide, which is applied in the field of new compound preparation, can solve the problems of inability to directly apply clinically and have low activity, and achieve the effects of improving kidney damage activity, reducing inflammatory response and reducing levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Synthesis of Geniposide Lead Compound - Geniposide
[0046] (1) Synthesis of Geniposide (1a)
[0047]
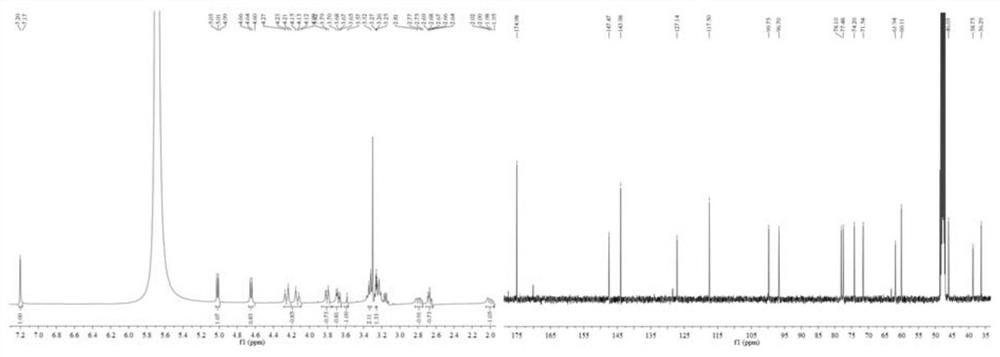
[0048] Geniposide (100.0 mg, 0.257 mmol) and 10 mL of 4 % NaOH solution were added to the round-bottomed flask in turn, and the reaction was stirred under reflux at 65 °C, and the reaction was followed by TLC until the starting point disappeared (developing agent v / v: Dichloromethane / methanol=5 / 1). After the reaction is completed, use 1M hydrochloric acid to neutralize to pH=7, purify, and concentrate the obtained reaction solution to dryness under reduced pressure, pass through the column (eluent v / v: dichloromethane / methanol=8 / 1-1 / 1), 61.5 mg of white powder was obtained, mp. 250.9-251.6 °C, yield 91.3%, which was determined to be geniposide (1a) by NMR, HR-MS and other analysis. Spectrum such as figure 1 shown.
[0049] 1 H NMR (400 MHz, CD 3 OD) δ 7.19 (d, J = 11.5 Hz, 1H), 5.01 (dd, J =9.1, 5.4 Hz, 1H), 4.64 (dd,J = 12.4, 8.4 Hz, 1H),...
Embodiment 2
[0050] Example 2 Synthesis of Geniposide Derivative Pentaacetyl Geniposide (2a)
[0051] (1) Synthesis of pentaacetylgeniposide (2a)
[0052]
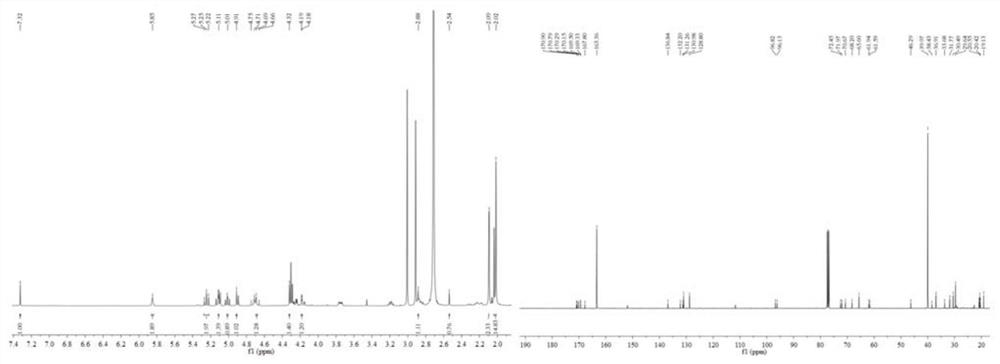
[0053] Geniposide derivative 1a (100.0 mg, 0.026 mmol) and 5 mL of triethylamine were added to the round-bottomed flask in turn, cooled in an ice-water bath, and 5 mL of acetic anhydride was slowly added dropwise with stirring. After the dropwise addition, the ice-water bath was removed. , the reaction was carried out at room temperature, and TLC was traced to the disappearance of the starting material point (developing solvent v / v: petroleum ether / ethyl acetate=1 / 1, 1 drop of formic acid). After the reaction, the reaction solution was washed with saturated NaHCO 3 The solution (20 mL) was neutralized to pH=7.0, washed with deionized water (20 mL × 3), the organic phases were combined, anhydrous Na 2 SO 4 Dry overnight and remove Na by suction filtration 2 SO 4 After distillation under reduced pressure, passed through the colum...
Embodiment 3 5
[0056] Example 3 Synthesis of pentaacetylgeniposide-3''-benzonitrile ester (5a)
[0057]
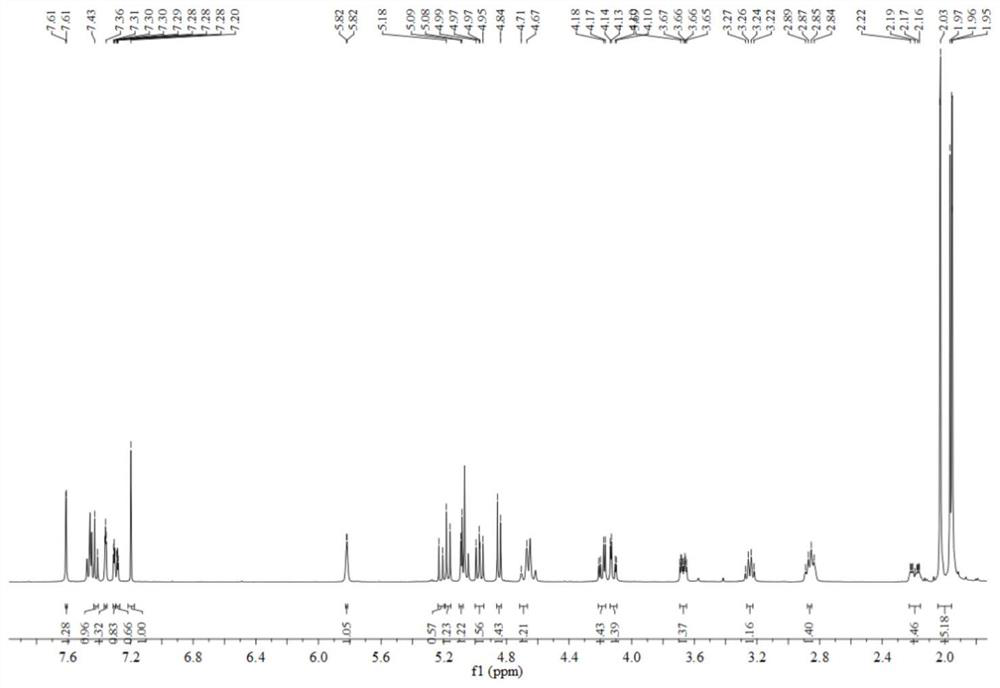
[0058] Geniposide derivative 2a (200.0 mg, 0.34 mmol) DMF (3 mL), EDCI (78.0 mg, 0.41 mmol) in a stirred solution, HOBT (55.0 mg, 0.41 mmol) and DIPEA (0.2 mL, 1.02 mmol) were added ), stirred at room temperature for 2 h, then added 3-hydroxybenzonitrile (44.0 mg, 0.37 mmol) and DMAP (63.0 mg, 0.51 mmol), and continued stirring at room temperature overnight. After the reaction was over, the solution used was added to ice water (30 mL) and extracted with dichloromethane (20 mL x 3). The combined organic solvent was washed with 1 M water diluted hydrochloric acid (20 mL × 3) and saturated brine (20 mL × 3). The organic phase is in anhydrous anhydrous Na 2 SO 4 It was dried overnight, distilled under reduced pressure, and purified by column chromatography (eluent v / v: petroleum ether / ethyl acetate=8 / 1-1 / 1) to obtain pale yellow powder 79.7 mg, mp.172.6-173.3 ℃, the yield was 34.1%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com