Oligomeric or polymerized methylene protein and its separation and purification process and use
A technology for separation and purification of proteins, applied in the field of protein preparation, can solve problems that have not yet been seen, and achieve the effects of protecting protein activity and separation and purification effects, reducing losses, and protecting integrity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 extracts alcohol dehydrogenase (tetramer protein) from yeast
[0032] Yeast: Commercially available dry baker's yeast (Gist-Brocades Yeast Co., Ltd., Netherlands) was dissolved in distilled water at a yeast concentration of 10% weight / volume (W / V) (dry weight), and 8 liters of yeast suspension was prepared. Stir for one hour at 4 °C to activate the yeast.
[0033] Adding protectant: After one hour, the cell suspension was divided into two parts (4 liters each), and 1 liter of glycerol and 20% (W / V) sucrose was added to one part of the activated yeast suspension under stirring. The solution was mixed to make the suspension volume 5 liters; another cell suspension without any protective agent was supplemented with distilled water to 5 liters as a reference comparison.
[0034] Broken cells: Use a high-pressure homogenizer pump produced by APV Manton Gaulin in the United States to add five liters of raw material solution into the feeding barrel and stir it. P...
Embodiment 2
[0036] Experimental results: the activity of alcohol dehydrogenase obtained without adding any protective agent is 25u / ml, where u represents the unit of enzyme activity, and / ml represents the slurry after per milliliter of cell disruption. Under the same experimental conditions, after adding glycerol and sucrose, the activity of alcohol dehydrogenase obtained is 40u / ml, which is 60% higher than that without protective agent. Example 2 Extracting Human Tumor Necrosis Factor (Trimer) from Genetically Engineered Escherichia coli
[0037] Escherichia coli (E.coli cell) culture: recombinant human tumor necrosis factor (rhTNF-α) Escherichia coli JM103, culture medium is 1.5% weight / volume (W / V)_tryptone, 1.3% weight / volume (W / V ) yeast extract powder, 0.25% weight / volume (W / V) NaCl, 0.2% weight / volume (W / V) glucose, pH 7.5. The final concentration of tetracycline is 12.5 μg / l. The fermentation of the recombinant bacteria was carried out at 37° C. with a 6-liter fermenter (product...
Embodiment 3
[0042] It can be seen from the above table that the addition of protective agents increases the activity of rhTNF-α by more than 30%, and PEG200 has the best effect, increasing the activity by more than 2 times. Example 3 Purification of Genetic Engineering Human Tumor Necrosis Factor by Adsorption
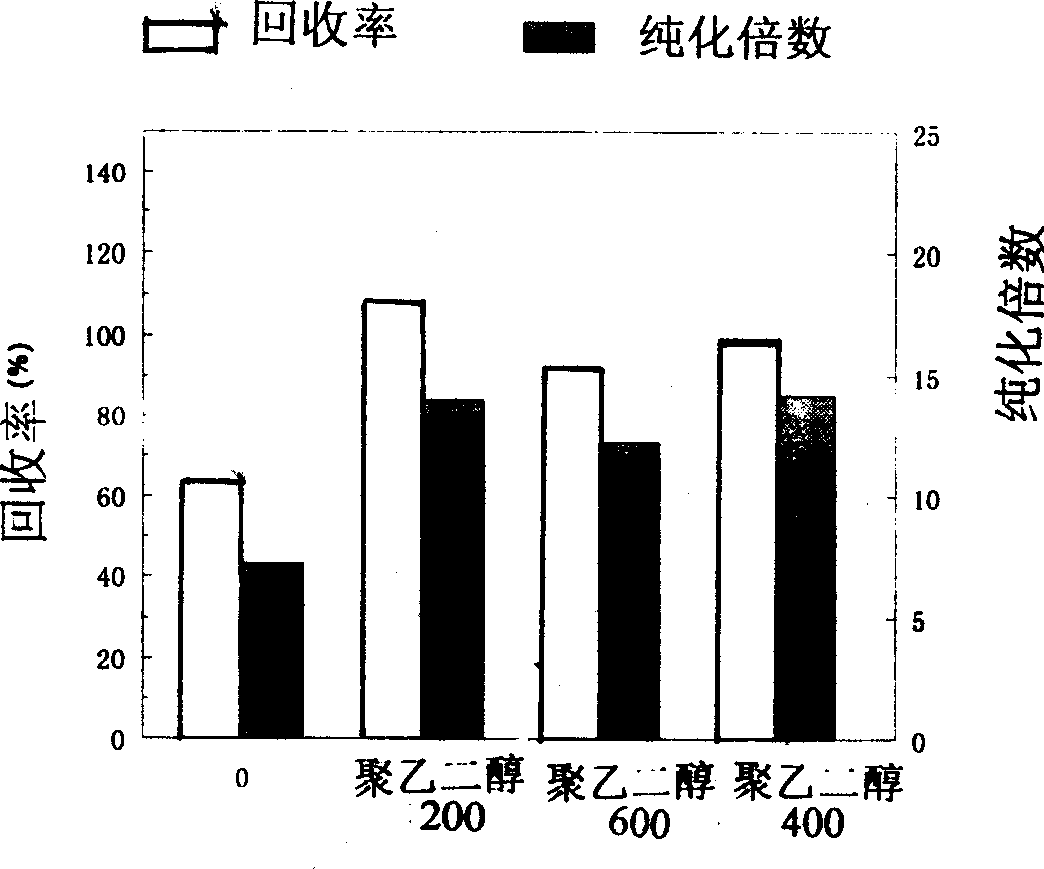
[0043] Adsorption: Anion-exchange medium DEAE-Sepharose Cl-6B is loaded into an adsorption column (16×55mm), fully equilibrated with 0.01M Tris-HCl, pH 8.0 (with or without polyethylene glycol PEG). Add polyethylene glycol PEG200, 600 or 4000 at a concentration of 1 wt% to the cell disruption supernatant, balance solution, and eluate without the presence of polyethylene glycol PEG, respectively. Feed, wash with equilibration buffer to remove unadsorbed protein to baseline, use 75mM NaCl, 10mM Tris-HCl for segmental elution, collect each elution peak and measure protein concentration and rhTNF-α activity.
[0044] Activity analysis: TNF activity analysis refers to the method of Ag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com