Preparation method for granula made of alpha-alcoholic acid resin ,and its use
A hydroxy acid and resin technology, applied in the field of resin particle preparation, can solve the problems of different release rates, difficulty in popularization and application, drug poisoning, etc., and achieve the effects of small burst release effect, wide application value, and stable drug release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
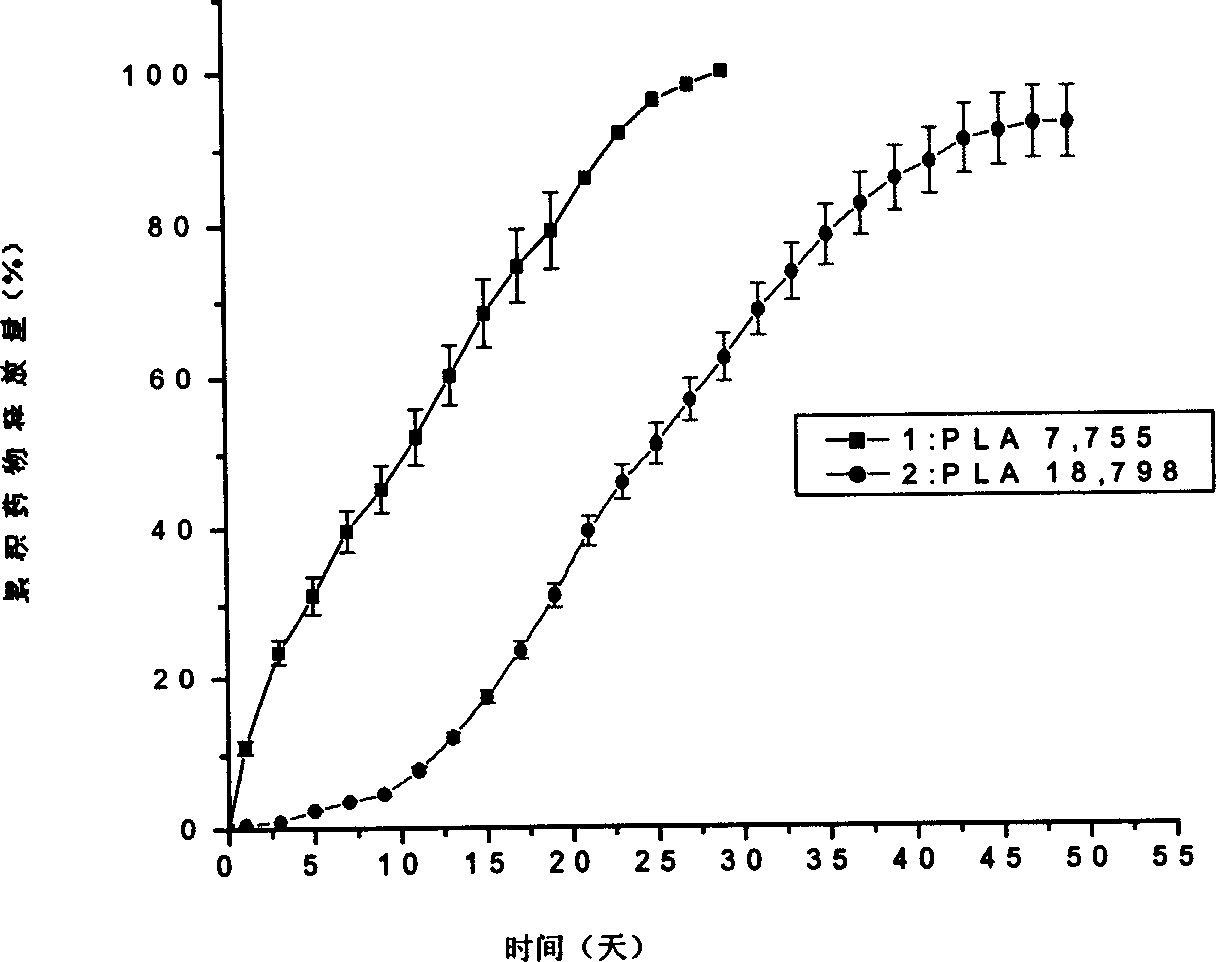
[0015] Implementation Example 1: Preparation of aspirin sustained-release microparticles with low molecular weight PLA
[0016] Prepare low-molecular-weight PLA drug-loaded microparticles with solvent evaporation method: take low-molecular-weight PLA (weight-average molecular weight M w 7,755) 30g was dissolved in 50mL of dichloromethane to make an oil phase resin solution; 908.1mg of type B gelatin was dissolved in 10mL of high-purity water to form a gelatin aqueous solution, and 1.5g of aspirin was suspended in the gelatin solution under magnetic stirring at a speed of 200rpm; The obtained solution was mixed with the oil-phase resin solution, and the mixture was ultrasonicated 5 times with an ultrasonic generator, each ultrasonic time was 60 s, and the ultrasonic output power was 20 W, to form a water-in-oil emulsion, and the obtained emulsion was cooled to 4 ° C in a refrigerator, Then add 500mL of 0.5% polyvinyl alcohol aqueous solution, and the resulting mixture is homoge...
Embodiment 2
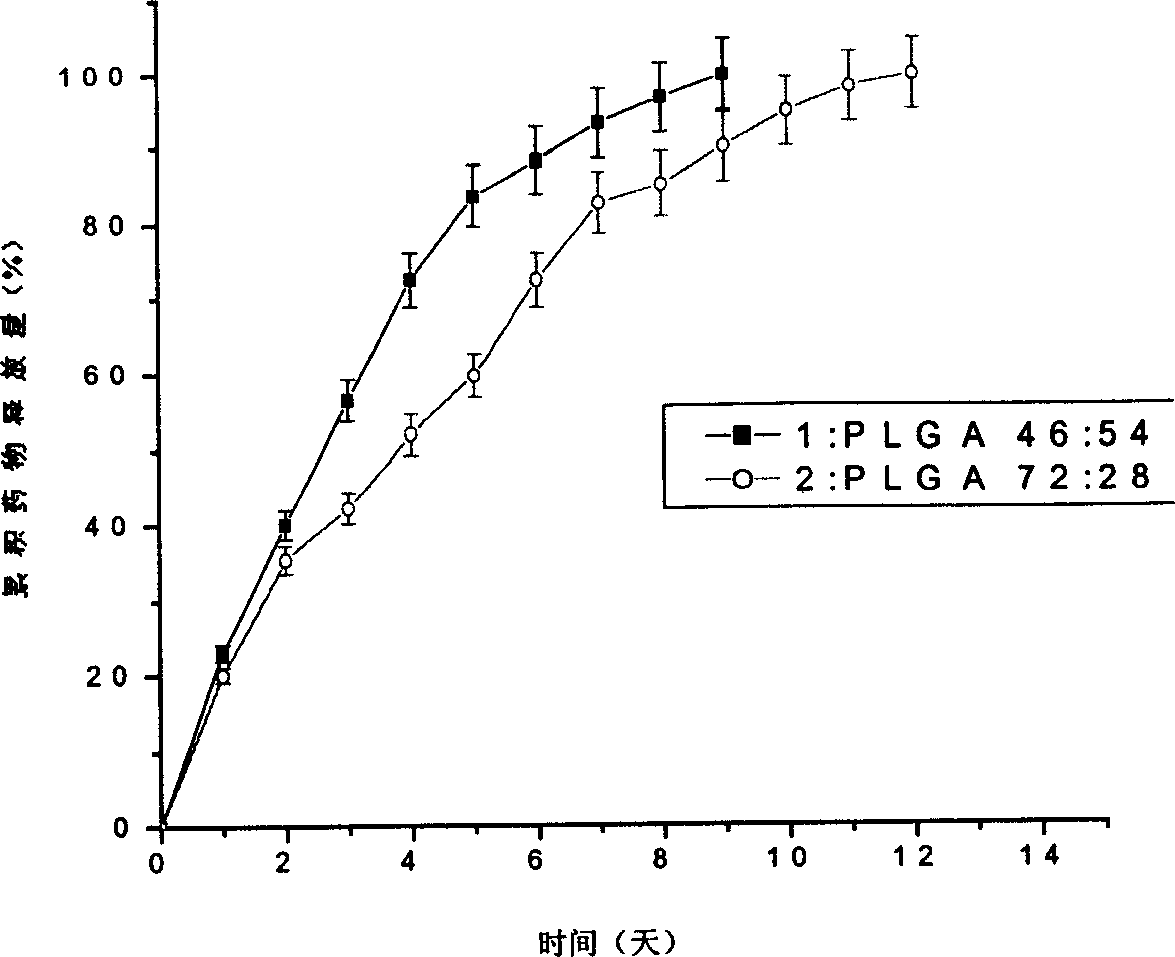
[0030] Implementation Example 2: Preparation of aspirin sustained-release microparticles with low molecular weight PLGA.
[0031] Preparation of drug-loaded particles by melting drug-loading method: Weigh two different lactic acid-glycolic acid low-molecular-weight copolymers (PLGA72 / 28, PLGA46 / 54, and the molar ratios of lactic acid and glycolic acid segments LA: GA are 72: 28, 46:54, weight average molecular weight M w 1368, 990 respectively). Weigh 5g of each sample, gradually soften into a liquid state in a silicone oil bath at 120°C, weigh 0.5g of aspirin, grind it in a mortar, add it to the liquid oligomer, keep the temperature of the mixture, and mix the drug with the liquid state with a glass stirring rod. The oligomer is mixed evenly, and the resulting mixture system is transferred to a cylindrical mold, and cooled to -5°C to mold the drug-loaded oligomer to form a rod; the obtained rod-shaped aspirin-loaded PLGA oligomer is used The glass pestle was ground in a mor...
Embodiment 3
[0035] Implementation Example 3: Bovine serum albumin controlled-release microparticles were prepared with a mixture of PLGA resins with different molecular weights.
[0036] Drug-loaded microparticles were prepared by solvent evaporation: two lactic acid-glycolic acid copolymers (PLGA: LA / GA75 / 25, Mw 10,091, 23.69g and PLGA: LA / GA75 / 25, Mw 1,715, 7.14g) were dissolved in Prepare an oil phase resin solution in 50 mL of dichloromethane; dissolve 908.1 mg of B-type gelatin in 10 mL of high-purity water to form a gelatin aqueous solution, and dissolve 1 g of bovine serum albumin in the gelatin solution; mix the obtained solution with the oil phase resin solution, and use ultrasonic The generator ultrasonicated the mixture 5 times, each ultrasonic time was 60s, and the ultrasonic output power was 20W to form a water-in-oil emulsion; the resulting emulsion was cooled to about 0°C in a refrigerator, and then added to 500mL of 0.5% polyvinyl alcohol aqueous solution , the resulting m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com